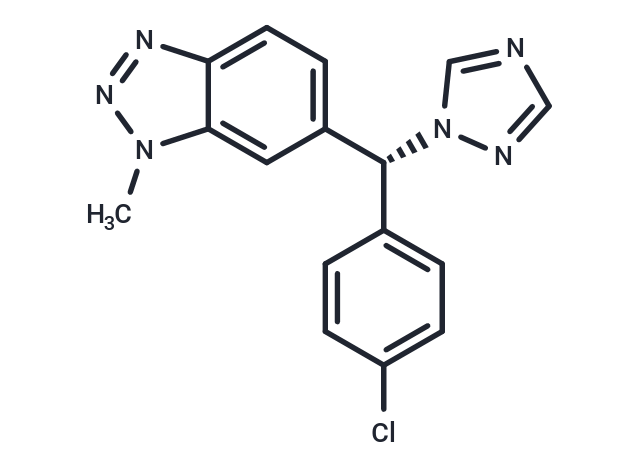
(-)-Vorozole
CAS No. 132042-69-4
(-)-Vorozole( —— )
Catalog No. M34141 CAS No. 132042-69-4
(-)-Vorozole is an orally active non-steroidal aromatase inhibitor with potency and selectivity.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 172 | In Stock |


|
| 10MG | 254 | In Stock |


|
| 25MG | 392 | In Stock |


|
| 50MG | 531 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name(-)-Vorozole
-
NoteResearch use only, not for human use.
-
Brief Description(-)-Vorozole is an orally active non-steroidal aromatase inhibitor with potency and selectivity.
-
Description(-)-Vorozole is an orally active non-steroidal aromatase inhibitor with potency and selectivity. (-)-Vorozole has demonstrated antitumor activity in in vivo experiments. (-)-Vorozole is used in the study of breast cancer.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayMetabolic Enzyme/Protease
-
TargetP450
-
RecptorP450 | Aromatase
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number132042-69-4
-
Formula Weight324.77
-
Molecular FormulaC16H13ClN6
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES[C@H](C=1C=C2C(=CC1)N=NN2C)(C3=CC=C(Cl)C=C3)N4C=NC=N4
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
AS1810722
AS1810722 is an orally active and potent STAT6 inhibitor(IC50 :1.9 nM). AS1810722 shows a good profile of CYP3A4 inhibition. AS1810722, a derivative of fused bicyclic pyrimidine, has the potential for allergic diseases such as asthma and atopic diseases research.
-
2,3-dihydrothieno-Th...
2,3-dihydrothieno-Thiadiazole Carboxylate is a CYP450 (CYP2E1 and CYP2B4) inhibitor.
-
Pregnenolone Carboni...
Pregnenolone Carbonitrile is an activator of rodent-PXR and induces the expression of CYP3A.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


