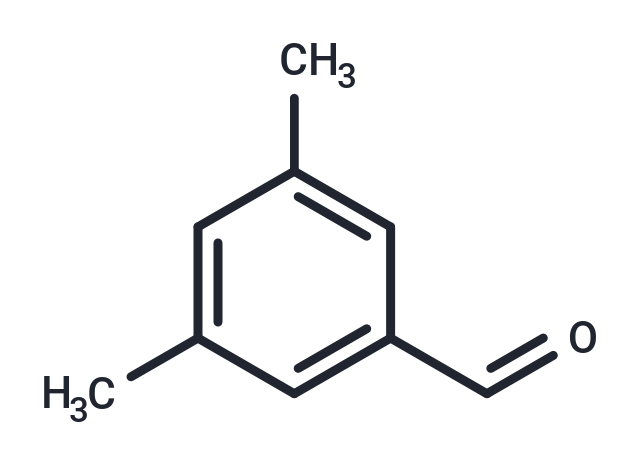
3,5-Dimethylbenzaldehyde
CAS No. 5779-95-3
3,5-Dimethylbenzaldehyde( —— )
Catalog No. M35010 CAS No. 5779-95-3
3,5-Dimethylbenzaldehyde has broad-spectrum antimicrobial activity, inhibiting Bacillus subtilis, Pseudomonas albicans, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 37 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | 28 | In Stock |


|
| 1G | 38 | In Stock |


|
Biological Information
-
Product Name3,5-Dimethylbenzaldehyde
-
NoteResearch use only, not for human use.
-
Brief Description3,5-Dimethylbenzaldehyde has broad-spectrum antimicrobial activity, inhibiting Bacillus subtilis, Pseudomonas albicans, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae.
-
Description3,5-Dimethylbenzaldehyde is a building block in the chemical synthesis.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorEndogenous Metabolite | Antifungal
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number5779-95-3
-
Formula Weight134.18
-
Molecular FormulaC9H10O
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (745.27 mM; Ultrasonic )
-
SMILESCC1=CC(C=O)=CC(C)=C1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. M Tudorie, et al. Coupled large amplitude motions: a case study of the dimethylbenzaldehyde isomers. J Phys Chem A. 2013 Dec 19;117(50):13636-47.?
molnova catalog



related products
-
DPPC
DPPC is a zwitterionic glycerophospholipid commonly used in the formation of lipid monolayers bilayers and liposomes.
-
Darusentan
Darusentan is a potent inhibitor of endothelin signaling and function in both large and small arteries.
-
Adrenic Acid
Adrenic acid is a member of the class of compounds known as very-long-chain fatty acids. Adrenic acid can be found in blood and in human myelin tissue.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


