SIBA
CAS No. 35899-54-8
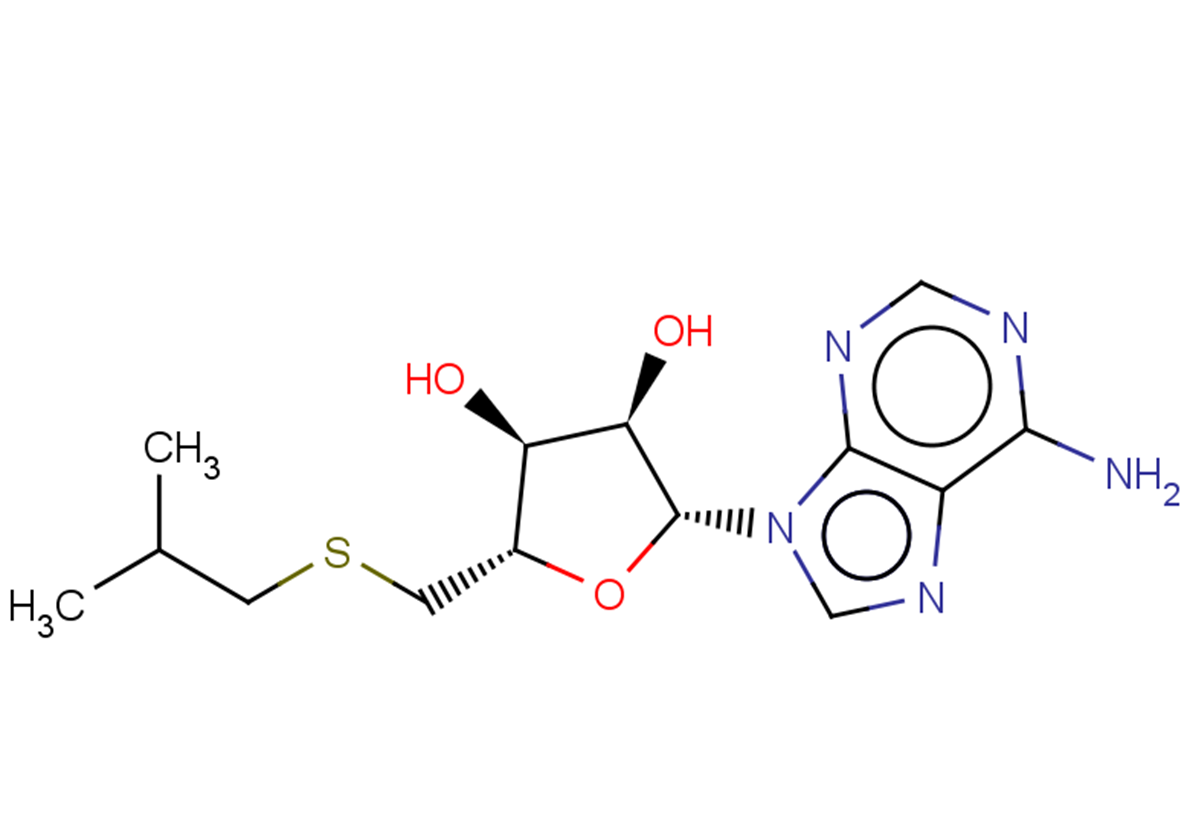
SIBA( 5'-Isobutylthioadenosine | 5'-Deoxy-5'-isobutylthioadenosine )
Catalog No. M24314 CAS No. 35899-54-8
SIBA is a synthetic analogue of SAH, acts as an inhibitor of S-adenosylmethionine-mediated transmethylation. SIBA can interfere with a variety of enzymatic activities in vitro, such as SAH hydrolase, methylthioadenosine phosphorylase and cAMP phosphodiesterase.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 101 | In Stock |


|
| 5MG | 90 | In Stock |


|
| 10MG | 113 | In Stock |


|
| 25MG | 219 | In Stock |


|
| 50MG | 322 | In Stock |


|
| 100MG | 458 | In Stock |


|
| 200MG | 639 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameSIBA
-
NoteResearch use only, not for human use.
-
Brief DescriptionSIBA is a synthetic analogue of SAH, acts as an inhibitor of S-adenosylmethionine-mediated transmethylation. SIBA can interfere with a variety of enzymatic activities in vitro, such as SAH hydrolase, methylthioadenosine phosphorylase and cAMP phosphodiesterase.
-
DescriptionSIBA is a synthetic analogue of SAH, acts as an inhibitor of S-adenosylmethionine-mediated transmethylation. SIBA can interfere with a variety of enzymatic activities in vitro, such as SAH hydrolase, methylthioadenosine phosphorylase and cAMP phosphodiesterase. SIBA reversibly blocks the multiplication of herpes simplex type 1 virus (HSV) .
-
In VitroSIBA (0.5 mM; 24-96 h) shows strong anti-proliferative activity against 3LL and RMS-J1 tumour cells.SIBA (1 mM; 12, 24 h) reversibly inhibits HSV production in HEp2 cells (infected by HSV-1).SIBA inhibits protein synthesis by 98% after 10 h infection of HEp2 cells (infected by HSV-1).SIBA (1 mM; 8.5 h) inhibits protein synthesis and RNA methylation in HEp2 cells (infected by HSV-1).SIBA (0.5, 1.0 mM; 24, 48 h) inhibits the conversion of putrescine into spermidine and/or spermine and that this inhibition is a reversible one (interferes with polyamine biosynthesis, probably by blocking aminopropyltransferase). Cell Proliferation Assay Cell Line:3 LL and RMS-J1 cells Concentration:0.5 mM Incubation Time:24-96 h Result:Inhibited 3LL and RMS-J1 tumor cell growth potently by 96% and 88%, respectively.Cell Viability Assay Cell Line:HEp2 cells (infected by HSV-1) Concentration:1 mM Incubation Time:12, 24 h Result:Decreased virus production by 88.4 and 98.2% when at 12 and 24 h, respectively.Cell Viability Assay Cell Line:HEp2 cells (infected by HSV-1) Concentration:1 mM Incubation Time:8.5 h Result:Reduced protein synthesis by 41.3% in normal medium and by 63.5% in medium poor in methionine.Inhibited RNA methylation by 65.4%.Cell Viability AssayCell Line:chick embryo fibroblastsConcentration:0.5, 1.0 mMIncubation Time:24, 48 h Result:Inhibited the uptake of the radioactive diamine and that the inhibition was dose-dependent.Markedly inhibited the formation of [14C]spermidine and [14C]spermine from [14C]putrescine.Inhibited the growth of chick embryo fibroblasts mainly after exposure for 48 h.
-
In VivoSIBA (150 mg/kg; i.p.; twice weekly for 3 weeks) inhibits tumor growth in vivo.SIBA (15 mg/kg; i.p.; thrice weekly for 4 weeks) inhibits metastatic spread of RMS-J1 cells in vivo. Animal Model:C57BL/6 female mice (4-8 weeks old).Dosage:150 mg/kg Administration:Intraperitoneal injection; twice weekly for 3 weeks.Result:Significantly reduced the median number of lung metastases.Animal Model:Adult syngeneic Wistar AG rats (8-week-old; subcutaneously grafted with RMS-J1 cells).Dosage:15 mg/kg Administration:Intraperitoneal injection; thrice weekly for 4 weeks.Result:Inhibited in vivo metastatic spread of RMS-J1 cells, and showed median numbers of lung metastatic nodules was 26.
-
Synonyms5'-Isobutylthioadenosine | 5'-Deoxy-5'-isobutylthioadenosine
-
PathwayMicrobiology/Virology
-
TargetHSV
-
RecptorHSV|Nucleoside Antimetabolite/Analog
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number35899-54-8
-
Formula Weight339.41
-
Molecular FormulaC14H21N5O3S
-
Purity>98% (HPLC)
-
SolubilityDMSO:90 mg/mL (265.17 mM)
-
SMILESCC(C)CSC[C@H]([C@H]([C@H]1O)O)O[C@H]1n1c2ncnc(N)c2nc1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Carteni-Farina M, et al. Studies on the metabolism of 5'-isobutylthioadenosine (SIBA): phosphorolytic cleavage by methylthioadenosinephosphorylase. FEBS Lett. 1979 Aug 15;104(2):266-270.
molnova catalog



related products
-
FV-100
FV-100 (Valnivudine, CF-1743) is a potent, selective, bicyclic nucleoside analogue inhibitor of varicella zoster virus (VZV) with EC50 of subnanomolar range in vitro.
-
B220
B220, an antiviral agent, inhibits the growth of HSV-1, HSV-2, and human cytomegalovirus (CMV).
-
nerolidol
Nerolidol shows sedative effects in animals, oxidative process plays a crucial role on neuronal pathological consequence.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


