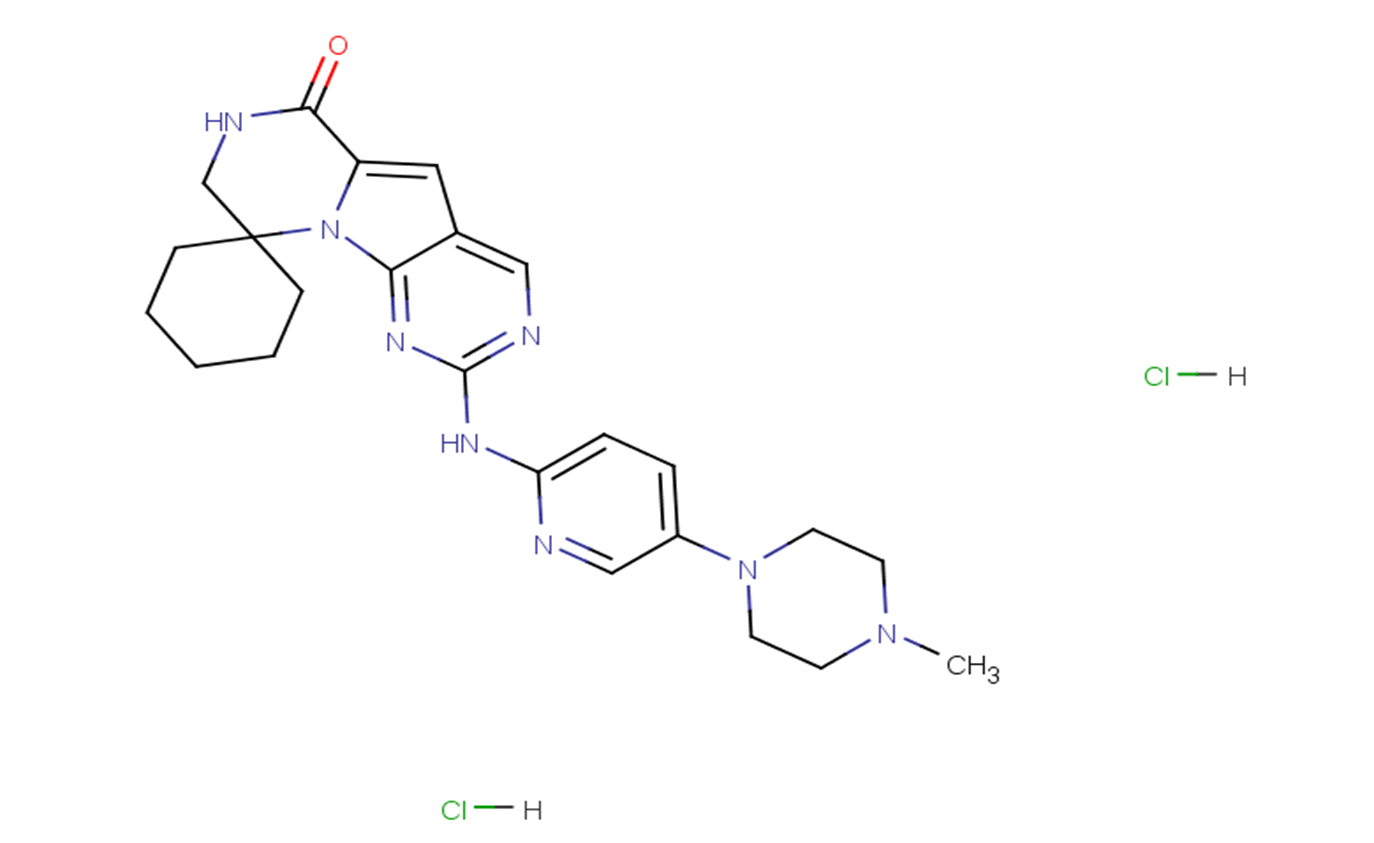
Trilaciclib hydrochloride
CAS No. 1977495-97-8
Trilaciclib hydrochloride( G1T28 hydrochloride )
Catalog No. M22845 CAS No. 1977495-97-8
Trilaciclib hydrochloride is an inhibitor of CDK4/6 (IC50s: 1 nM and 4 nM for CDK4 and CDK6).Incubation with Trilaciclib hydrochloride (G1T28) for 24 hours can induce a strong G1 cell cycle arrest (time=0). Cells have re-entered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells By 16 hours after Trilaciclib hydrochloride washout.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 81 | In Stock |


|
| 10MG | 147 | In Stock |


|
| 25MG | 305 | In Stock |


|
| 50MG | 512 | In Stock |


|
| 100MG | 745 | In Stock |


|
| 200MG | 995 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameTrilaciclib hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionTrilaciclib hydrochloride is an inhibitor of CDK4/6 (IC50s: 1 nM and 4 nM for CDK4 and CDK6).Incubation with Trilaciclib hydrochloride (G1T28) for 24 hours can induce a strong G1 cell cycle arrest (time=0). Cells have re-entered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells By 16 hours after Trilaciclib hydrochloride washout.
-
DescriptionTrilaciclib hydrochloride is an inhibitor of CDK4/6 (IC50s: 1 nM and 4 nM for CDK4 and CDK6).Incubation with Trilaciclib hydrochloride (G1T28) for 24 hours can induce a strong G1 cell cycle arrest (time=0). Cells have re-entered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells By 16 hours after Trilaciclib hydrochloride washout. These results demonstrate that Trilaciclib hydrochloride causes a transient, and reversible G1 arrest. A transient Trilaciclib hydrochloride-mediated G1 cell-cycle arrest in CDK4/6-sensitive cells decreases the in vitro toxicity of a variety of commonly used cytotoxic chemotherapy agents associated with myelosuppression.G1T28 inhibits the phosphorylation of RB and induces an exclusive, reversible G1 arrest.n vivo, G1T28 reversibly and in a dose-dependent manner, regulates the proliferation of HSPCs. Pretreatment of mice with oral G1T28 allows for the faster recovery of CBCs following chemotherapy treatment. Likewise, oral G1T28 does not protect RB-deficient tumors from chemotherapy but adds to the antitumor effect. Although this effect was found in athymic mice that lack T lymphocytes, it is still possible that the enhanced efficacy is due to preservation of other immune cell types such as natural killer cells. (In Vitro):Incubation with Trilaciclib hydrochloride (G1T28) for 24 hours induces a robust G1 cell-cycle arrest (time=0). By 16 hours after Trilaciclib hydrochloride washout, cells have reentered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells. These results demonstrate that Trilaciclib hydrochloride causes a transient, and reversible G1 arrest. A transient Trilaciclib hydrochloride-mediated G1 cell-cycle arrest in CDK4/6-sensitive cells decreases the in vitro toxicity of a variety of commonly used cytotoxic chemotherapy agents associated with myelosuppression. (In Vivo):Trilaciclib hydrochloride (G1T28) treatment results in a robust and dose-dependent suppression of proliferation in HSPCs at 12 hours, with 5-ethynyl-2′-deoxyuridine (EdU) incorporation returning near baseline levels in a dose-dependent manner by 24 hours after administration. These data demonstrate that a single oral dose of Trilaciclib hydrochloride can produce reversible cell-cycle arrest in HSPCs in a dose-dependent manner in vivo. Mice given 100 mg/kg Trilaciclib hydrochloride 30 minutes prior to etoposide treatment, exhibits only background levels of caspase-3/7 activity. These data demonstrate that Trilaciclib hydrochloride can protect the bone marrow from chemotherapy-induced apoptosis in vivo. The data demonstrate that treatment with Trilaciclib hydrochloride prior to 5-fluorouracil (5-FU) likely decreases 5-FU-induced damage by chemotherapy in HSPCs, thus accelerating blood count recovery after chemotherapy.
-
In VitroIncubation with Trilaciclib hydrochloride (G1T28) for 24 hours induces a robust G1 cell-cycle arrest (time=0). By 16 hours after Trilaciclib hydrochloride washout, cells have reentered the cell cycle and demonstrate cell-cycle kinetics similar to untreated control cells. These results demonstrate that Trilaciclib hydrochloride causes a transient, and reversible G1 arrest. A transient Trilaciclib hydrochloride-mediated G1 cell-cycle arrest in CDK4/6-sensitive cells decreases the in vitro toxicity of a variety of commonly used cytotoxic chemotherapy agents associated with myelosuppression.
-
In VivoTrilaciclib hydrochloride (G1T28) treatment results in a robust and dose-dependent suppression of proliferation in HSPCs at 12 hours, with 5-ethynyl-2′-deoxyuridine (EdU) incorporation returning near baseline levels in a dose-dependent manner by 24 hours after administration. These data demonstrate that a single oral dose of Trilaciclib hydrochloride can produce reversible cell-cycle arrest in HSPCs in a dose-dependent manner in vivo. Mice given 100 mg/kg Trilaciclib hydrochloride 30 minutes prior to etoposide treatment, exhibits only background levels of caspase-3/7 activity. These data demonstrate that Trilaciclib hydrochloride can protect the bone marrow from chemotherapy-induced apoptosis in vivo. The data demonstrate that treatment with Trilaciclib hydrochloride prior to 5-fluorouracil (5-FU) likely decreases 5-FU-induced damage by chemotherapy in HSPCs, thus accelerating blood count recovery after chemotherapy.
-
SynonymsG1T28 hydrochloride
-
PathwayAngiogenesis
-
TargetCDK
-
RecptorCdk4/cyclin D1|cdk6/cyclin D3
-
Research Areacancer
-
IndicationColorectal Cancer Metastatic|Myelosuppression Adult|Chemotherapeutic Toxicity
Chemical Information
-
CAS Number1977495-97-8
-
Formula Weight519.47
-
Molecular FormulaC24H32Cl2N8O
-
Purity>98% (HPLC)
-
SolubilityH2O:25.64 mg/mL (49.36 mM; ultrasonic and adjust pH to 2 with HCl); DMSO:1.1 mg/mL (2.12 mM; Need ultrasonic)
-
SMILESO=C1C2=CC3=CN=C(NC4=CC=C(N5CCN(C)CC5)C=N4)N=C3N2C6(CCCCC6)CN1.[H]Cl.[H]Cl
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Bisi JE, et al. Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol Cancer Ther. 2016 May;15(5):783-93.
molnova catalog



related products
-
NU-6300
NU-6300 is the first covalent, irreversible, ATP-Competitive CDK2 inhibitor with Ki of 6 nM.
-
Purvalanol A
A potent, cell-permeable, selective inhibitor of CDK with IC50s of 4, 70, 35, 850, and 75 nM for cdc2/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, Cdk4/cyclin D1 and Cdk5-p35, respectively.
-
XY028-140
XY028-140 is a selective CDK4/CDK6 degrade and inhibits both CDK4/6 expression and activity in cancer cells. XY028-140 is a PROTAC connected by ligands for Cereblon and CDK.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


