Lexibulin
CAS No. 917111-44-5
Lexibulin( CYT-997 )
Catalog No. M16586 CAS No. 917111-44-5
An orally active tubulin polymerization inhibitor with potent cytotoxic and vascular disrupting activity in vitro and in vivo.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 87 | In Stock |


|
| 10MG | 165 | In Stock |


|
| 25MG | 331 | In Stock |


|
| 50MG | 509 | In Stock |


|
| 100MG | 732 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameLexibulin
-
NoteResearch use only, not for human use.
-
Brief DescriptionAn orally active tubulin polymerization inhibitor with potent cytotoxic and vascular disrupting activity in vitro and in vivo.
-
DescriptionAn orally active tubulin polymerization inhibitor with potent cytotoxic and vascular disrupting activity in vitro and in vivo; inhibits a broad selection of cancer cell lines with IC50 of <100 nM; blocks the cell cycle at the G2-M boundary and increases phosphorylated Bcl-2, expression of cyclin B1; orally bioavailable, and efficacious in a range of in vivo cancer models.Blood Cancer Phase 2 Discontinued(In Vitro):Lexibulin (CYT-997) prevents the in vitro polymerization of tubulin with an IC50 of ~3 μmol/L (compared with the half-maximal inhibitory concentration of 2 μmol/L for colchicine under identical conditions) as determined using the conventional turbidimetric assay for tubulin polymerization. Lexibulin is also capable of reversibly disrupting the microtubule network in cells, visualized using fluorescence microscopy. Thus, treatment of A549 cells with Lexibulin (1 μM) lead to the rapid reorganization of microtubules, including the destruction of the existing microtubule network and accumulation of tubulin in plaques within the cytoplasm of some cells. After 24 hours, major alterations in cell morphology are evident, including loss of adhesion and cell rounding. The effect of 1 hour of treatment with Lexibulin is reversible and cells rapidly recovered their normal microtubule architecture. Taken together, the data indicates that Lexibulin belongs to the class of anticancer agents that disrupt, rather than stabilize, tubulin-containing structures. Although vehicle-treated cells show 15% and 19% in G2-M phase at 15 and 24 hours (respectively), cells treated with Lexibulin (1 μM) had 38% and 43% of cells in G2-M at the same time points. Furthermore, at 24 hours post-Lexibulin treatment, only 66% of total cells are in the G1, S, and G2-M phases, which suggests that cells blocked at the G2-M boundary do not exit back to G1, as in the normal cell cycle, but most likely are driven towards apoptosis and cell death. Consistent with the disruption of cellular tubulin, Lexibulin potently inhibits proliferation, induces cell cycle arrest and most importantly apoptosis of both human myeloma cell lines (HMCLs) and primary MM cells. (In Vivo):iIn a xenograft model using the human prostate cancer cell line PC3, oral dosing of Lexibulin (CYT-997) is initiated 13 days after cell implantation by which time palpable tumors were evident. A dose-dependent inhibition of tumor growth was apparent with Lexibulin (CYT-997), which at the highest dose was equivalent to parenterally administered paclitaxel. A single dose of Lexibulin (CYT-997) (7.5 mg/kg i.p.) clearly decreased blood flow in liver metastases, and a significant reduction in blood flow was present 6 hours postdose. Lexibulin (CYT-997) treatment (15 mg/kg/day) significantly prolongs the survival in a murine model of aggressive systemic myelomatosis.
-
In VitroLexibulin (CYT-997) prevents the in vitro polymerization of tubulin with an IC50 of ~3 μmol/L (compared with the half-maximal inhibitory concentration of 2 μmol/L for colchicine under identical conditions) as determined using the conventional turbidimetric assay for tubulin polymerization. Lexibulin is also capable of reversibly disrupting the microtubule network in cells, visualized using fluorescence microscopy. Thus, treatment of A549 cells with Lexibulin (1 μM) lead to the rapid reorganization of microtubules, including the destruction of the existing microtubule network and accumulation of tubulin in plaques within the cytoplasm of some cells. After 24 hours, major alterations in cell morphology are evident, including loss of adhesion and cell rounding. The effect of 1 hour of treatment with Lexibulin is reversible and cells rapidly recovered their normal microtubule architecture. Taken together, the data indicates that Lexibulin belongs to the class of anticancer agents that disrupt, rather than stabilize, tubulin-containing structures. Although vehicle-treated cells show 15% and 19% in G2-M phase at 15 and 24 hours (respectively), cells treated with Lexibulin (1 μM) had 38% and 43% of cells in G2-M at the same time points. Furthermore, at 24 hours post-Lexibulin treatment, only 66% of total cells are in the G1, S, and G2-M phases, which suggests that cells blocked at the G2-M boundary do not exit back to G1, as in the normal cell cycle, but most likely are driven towards apoptosis and cell death. Consistent with the disruption of cellular tubulin, Lexibulin potently inhibits proliferation, induces cell cycle arrest and most importantly apoptosis of both human myeloma cell lines (HMCLs) and primary MM cells.
-
In VivoiIn a xenograft model using the human prostate cancer cell line PC3, oral dosing of Lexibulin (CYT-997) is initiated 13 days after cell implantation by which time palpable tumors were evident. A dose-dependent inhibition of tumor growth was apparent with Lexibulin (CYT-997), which at the highest dose was equivalent to parenterally administered paclitaxel. A single dose of Lexibulin (CYT-997) (7.5 mg/kg i.p.) clearly decreased blood flow in liver metastases, and a significant reduction in blood flow was present 6 hours postdose. Lexibulin (CYT-997) treatment (15 mg/kg/day) significantly prolongs the survival in a murine model of aggressive systemic myelomatosis.
-
SynonymsCYT-997
-
PathwayCytoskeleton/Cell Adhesion Molecules
-
TargetMicrotubule/Tubulin
-
Recptormicrotubule
-
Research AreaCancer
-
IndicationBlood cancer
Chemical Information
-
CAS Number917111-44-5
-
Formula Weight434.534
-
Molecular FormulaC24H30N6O2
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
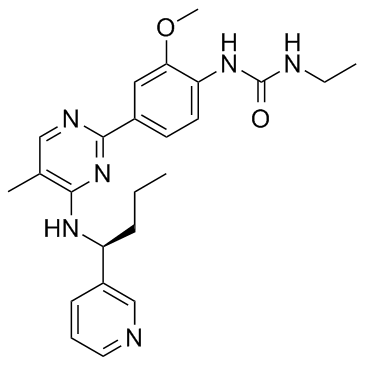
SMILESO=C(NC1=CC=C(C2=NC=C(C)C(N[C@H](C3=CC=CN=C3)CCC)=N2)C=C1OC)NCC
-
Chemical NameUrea, N-ethyl-N'-[2-methoxy-4-[5-methyl-4-[[(1S)-1-(3-pyridinyl)butyl]amino]-2-pyrimidinyl]phenyl]-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Burns CJ, et al. Mol Cancer Ther. 2009 Nov;8(11):3036-45.
2. Burns CJ, et al. Bioorg Med Chem Lett. 2009 Aug 15;19(16):4639-42.
3. Burns CJ, et al. J Pharmacol Exp Ther. 2011 Dec;339(3):799-806.
molnova catalog



related products
-
Ombrabulin
A novel combretastatin A-4 derivative and tubulin polymerization inhibitor that strongly stanches tumour blood flows and inhibits growth of tumours developing in various tissues and organ.
-
Taccalonolide B
Taccalonolide B is effective in vitro against cell lines that overexpress P-glycoprotein (Pgp) and multidrug-resistance protein (MRP7).?Taccalonolide B inhibits growth of SK-OV-3 cells with an IC50 of 208 nM.The structures were elucidated using a combination of spectroscopic methods, including 1D and 2D NMR and HR-ESI-MS.
-
BNC105
BNC105 is a tubulin polymerization inhibitor. It has potent antiproliferative and tumor vascular disrupting properties.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


