Tegobuvir
CAS No. 1000787-75-6
Tegobuvir( GS 333126 | GS 9190 | GS333126 | GS9190 )
Catalog No. M10009 CAS No. 1000787-75-6
A nonnucleoside inhibitor of HCV NS5B polymerase that acts as a potent inhibitor of HCV genotype 1.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 219 | Get Quote |


|
| 10MG | 372 | Get Quote |


|
| 25MG | 619 | Get Quote |


|
| 50MG | 881 | Get Quote |


|
| 100MG | 1188 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameTegobuvir
-
NoteResearch use only, not for human use.
-
Brief DescriptionA nonnucleoside inhibitor of HCV NS5B polymerase that acts as a potent inhibitor of HCV genotype 1.
-
DescriptionA nonnucleoside inhibitor of HCV NS5B polymerase that acts as a potent inhibitor of HCV genotype 1; inhibits 1a (H77) and 1b (Con-1) replicons with EC50 of 13.8 nM and 0.8 nM respectively, and displays a loss in potency against 2a (JFH-1) virus with EC50 of 2.9 uM.(In Vitro):Tegobuvir rapidly increases the proportion of replicons with the Y448H mutation in a dose-dependent manner. After 3 days of treatment, 1.2%, 6.8%, and > 50% of the replicon population expresses Y448H with the use of Tegobuvir at 1, 10, and 20 times its 50% effective concentration, respectively. Tegobuvir exerts anti-HCV activity utilizing a unique chemical activation and subsequent direct interaction with the NS5B protein. Treatment of HCV subgenomic replicon cells with Tegobuvir results in a modified form of NS5B with a distinctly altered mobility on a SDS-PAGE gel. Tegobuvir is potent in GT1a and 1b with mean EC50s of 19.8 and 1.5 nM respectively. For genotype 3a, 4a, and 6a Con chimeras, tegobuvir EC50s are all greater than 100 nM. The F445C NS5B mutations in GT3a, 4a, and 6a chimeric replicons restore tegobuvir potency to EC50 levels comparable to GT1a.
-
In VitroTegobuvir rapidly increases the proportion of replicons with the Y448H mutation in a dose-dependent manner. After 3 days of treatment, 1.2%, 6.8%, and > 50% of the replicon population expresses Y448H with the use of Tegobuvir at 1, 10, and 20 times its 50% effective concentration, respectively. Tegobuvir exerts anti-HCV activity utilizing a unique chemical activation and subsequent direct interaction with the NS5B protein. Treatment of HCV subgenomic replicon cells with Tegobuvir results in a modified form of NS5B with a distinctly altered mobility on a SDS-PAGE gel. Tegobuvir is potent in GT1a and 1b with mean EC50s of 19.8 and 1.5 nM respectively. For genotype 3a, 4a, and 6a Con chimeras, tegobuvir EC50s are all greater than 100 nM. The F445C NS5B mutations in GT3a, 4a, and 6a chimeric replicons restore tegobuvir potency to EC50 levels comparable to GT1a.
-
In Vivo——
-
SynonymsGS 333126 | GS 9190 | GS333126 | GS9190
-
PathwayMicrobiology/Virology
-
TargetHCV
-
RecptorHCV
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number1000787-75-6
-
Formula Weight517.401
-
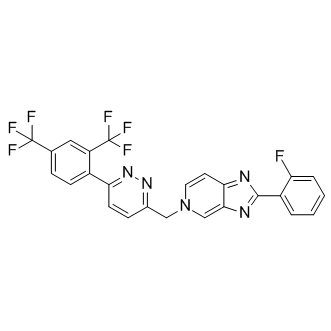
Molecular FormulaC25H14F7N5
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
SMILESFC1=CC=CC=C1C2=NC3=CN(CC4=NN=C(C5=CC=C(C(F)(F)F)C=C5C(F)(F)F)C=C4)C=CC3=N2
-
Chemical Name5H-Imidazo[4,5-c]pyridine, 5-[[6-[2,4-bis(trifluoromethyl)phenyl]-3-pyridazinyl]methyl]-2-(2-fluorophenyl)-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Shih IH, et al. Antimicrob Agents Chemother. 2011 Sep;55(9):4196-203.
2. Zeuzem S, et al. Hepatology. 2012 Mar;55(3):749-58.
3. Wong KA, et al. Virology. 2012 Jul 20;429(1):57-62.
molnova catalog



related products
-
HCV-IN-31
HCV-IN-31 is a HCV inhibitor with an EC50/EC95 of 15.7 μM.
-
RO8191
RO8191 (CDM-3008), an imidazonaphthyridine compound, is an agonist of interferon (IFN) receptor.
-
BMS-961955
BMS-961955 is an potent, allosteric inhibitor of HCV NS5B polymerase with EC50 of 7.9/4.3 nM for GT 1b/1a replicon respectively.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


