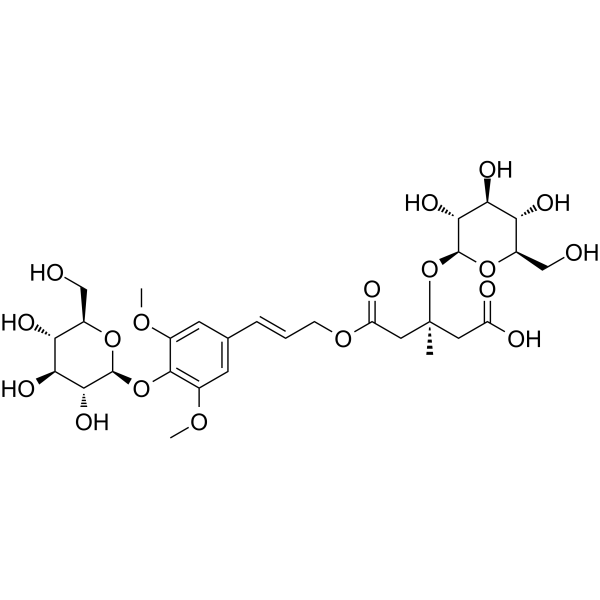
Tangshenoside I
CAS No. 117278-74-7
Tangshenoside I( —— )
Catalog No. M31829 CAS No. 117278-74-7
Tangshenoside I might be a potential bioactive marker related to the hematopoietic and immunologic functions of Codonopsis Radix, which could be recommended as the index compound. It has α-glucosidase inhibition activity.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 485 | In Stock |


|
| 50MG | Get Quote | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameTangshenoside I
-
NoteResearch use only, not for human use.
-
Brief DescriptionTangshenoside I might be a potential bioactive marker related to the hematopoietic and immunologic functions of Codonopsis Radix, which could be recommended as the index compound. It has α-glucosidase inhibition activity.
-
DescriptionTangshenoside I might be a potential bioactive marker related to the hematopoietic and immunologic functions of Codonopsis Radix, which could be recommended as the index compound. It has α-glucosidase inhibition activity.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number117278-74-7
-
Formula Weight678.6
-
Molecular FormulaC29H42O18
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?H2O : 25 mg/mL (36.84 mM)
-
SMILES——
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
4-O-Methylochnaflavo...
4'-O-Methylochnaflavone is a biflavonoid isolated from Lonicera japonica, suppresses mouse lymphocyte proliferation.
-
2F-Peracetyl-Fucose
2F-Peracetyl-Fucose (1,3,4-Tri-O-acetyl-2-deoxy-2-fluoro-L-fucopyranos) is a potent fucosyltransferase (FUT) inhibitor that suppresses salivary acidification and fucosylation in an in vitro inflammatory model.
-
A-G-D-V
A-G-D-V



 Cart
Cart
 sales@molnova.com
sales@molnova.com


