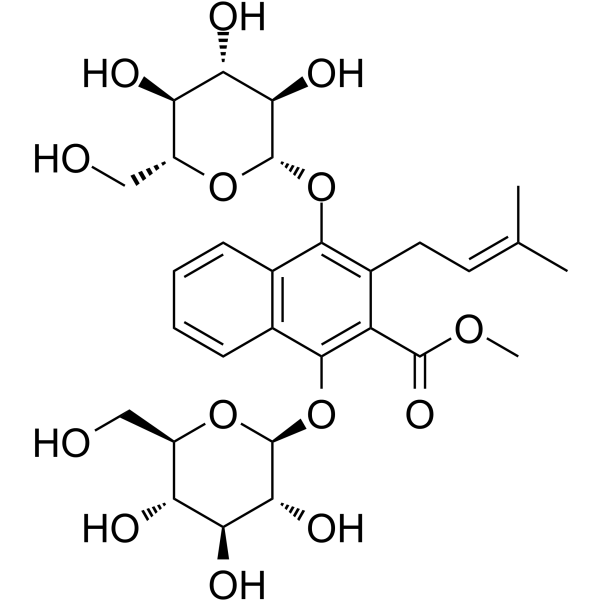
Methyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate
CAS No. 90685-26-0
Methyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate( —— )
Catalog No. M31577 CAS No. 90685-26-0
Methyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate is a natural product.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 144 | In Stock |


|
| 50MG | Get Quote | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameMethyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate
-
NoteResearch use only, not for human use.
-
Brief DescriptionMethyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate is a natural product.
-
DescriptionMethyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate is a natural product.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number90685-26-0
-
Formula Weight610.6
-
Molecular FormulaC29H38O14
-
Purity>98% (HPLC)
-
Solubility——
-
SMILES——
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
[Pyr1]-Apelin-13
[Pyr1]-Apelin-13 is a potent and selective endogenous Apelin receptor (APJ) agonist.[Pyr1]-apelin-13 encapsulation in lipoPEG particles (lipoPEG-PA13) results in sustained and extended drug release under physiological conditions[1].[Pyr1]-apelin-13 nanocarriers in a mouse model of pressure-overload induced heart failure demonstrate a sustainable long-term effect of [Pyr1]-apelin-13 in preventing cardiac dysfunction.
-
Biotin-Obestatin (hu...
Biotin-Obestatin (human)
-
Dexrazoxane
A derivative of EDTA that chelates iron and reduces the number of metal ions complexed with anthracycline and, consequently, decrease the formation of superoxide radicals.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


