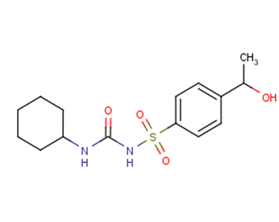
Hydroxyhexamide
CAS No. 3168-01-2
Hydroxyhexamide( (±)-Hydroxyhexamid )
Catalog No. M17724 CAS No. 3168-01-2
Hydroxyhexamide is a pharmacologically active metabolite of Acetohexamide, used as a hypoglycemic agent.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 75 | In Stock |


|
| 2MG | 81 | In Stock |


|
| 5MG | 133 | In Stock |


|
| 10MG | 237 | In Stock |


|
| 25MG | 402 | In Stock |


|
| 50MG | 581 | In Stock |


|
| 100MG | 799 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | 1611 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameHydroxyhexamide
-
NoteResearch use only, not for human use.
-
Brief DescriptionHydroxyhexamide is a pharmacologically active metabolite of Acetohexamide, used as a hypoglycemic agent.
-
DescriptionHydroxyhexamide is a pharmacologically active metabolite of Acetohexamide, used as a hypoglycemic agent.
-
In Vitro——
-
In Vivo——
-
Synonyms(±)-Hydroxyhexamid
-
PathwayMetabolic Enzyme/Protease
-
TargetPPAR
-
RecptorATP-sensitive potassium (KATP) channels
-
Research AreaCardiovascular Disease
-
Indication——
Chemical Information
-
CAS Number3168-01-2
-
Formula Weight326.41
-
Molecular FormulaC15H22N2O4S
-
Purity>98% (HPLC)
-
SolubilityDMSO : ≥ 300 mg/mL; 919.09 mM
-
SMILESO=C(NC1CCCCC1)NS(=O)(=O)c2ccc(cc2)C(C)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Imamura Y,etal.Hypoglycemic effect of S(-)-hydroxyhexamide, a major metabolite of acetohexamide, and its enantiomer R(+)-hydroxyhexamide.Life Sci. 2001 Sep 7;69(16):1947-55.
molnova catalog



related products
-
DG172 (dihydrochlori...
DG172 (dihydrochloride) is an ?antagonist of ?PPARβ/δ(IC50 : 27 nM).
-
Daidzein
Daidzein is a natural isoflavone phytoestrogen found in Leguminosae, used as a component of foods and dietary supplements.
-
Tetradecylthioacetic...
Tetradecylthioacetic acid is a synthetic fatty acid with a sulfur substitution in the β-position. This modification renders TTA unable to undergo complete β-oxidation and increases its biological activity, including activation of peroxisome proliferator activated receptors (PPARs) with preference for PPARα.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


