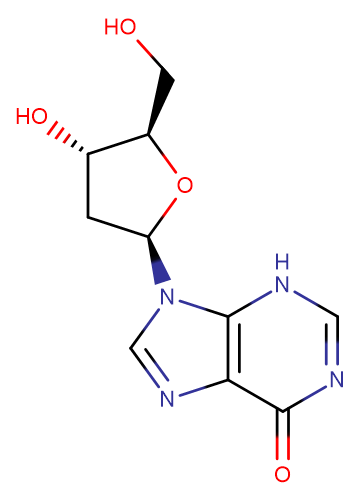
2'-Deoxyinosine
CAS No. 890-38-0
2'-Deoxyinosine( —— )
Catalog No. M16427 CAS No. 890-38-0
it is used to treat HIV infection with other antiviral drugs.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | 27 | In Stock |


|
Biological Information
-
Product Name2'-Deoxyinosine
-
NoteResearch use only, not for human use.
-
Brief Descriptionit is used to treat HIV infection with other antiviral drugs.
-
Descriptionit is used to treat HIV infection with other antiviral drugs. (In Vitro):In the absence of deoxycoformycin, 2’-deoxyadenosine is primarily deaminated to 2’-deoxyinosine and then converted into hypoxanthine. In the presence of the inhibitor, the deoxynucleoside, in addition to a phosphorylation process, undergoes phosphorolytic cleavage giving rise to adenine. The conversion of 2’-deoxyadenosine to adenine might represent a protective device, emerging when the activity of adenosine deaminase is reduced or inhibited. There is much evidence to indicate that the enzyme catalyzing this processs may be distinct from methylthioadenosine phosphorylase and S-adenosyl homocysteine hydrolase, which are the enzymes reported to be responsible for the formation of adenine from 28-deoxyadenosine in mammals.
-
In VitroIn the absence of deoxycoformycin, 2’-deoxyadenosine is primarily deaminated to 2’-deoxyinosine and then converted into hypoxanthine. In the presence of the inhibitor, the deoxynucleoside, in addition to a phosphorylation process, undergoes phosphorolytic cleavage giving rise to adenine. The conversion of 2’-deoxyadenosine to adenine might represent a protective device, emerging when the activity of adenosine deaminase is reduced or inhibited. There is much evidence to indicate that the enzyme catalyzing this processs may be distinct from methylthioadenosine phosphorylase and S-adenosyl homocysteine hydrolase, which are the enzymes reported to be responsible for the formation of adenine from 28-deoxyadenosine in mammals.
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number890-38-0
-
Formula Weight252.23
-
Molecular FormulaC10H12N4O4
-
Purity>98% (HPLC)
-
SolubilityLimited solubility
-
SMILESC1[C@@H]([C@H](O[C@H]1N2C=NC3=C2NC=NC3=O)CO)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Baumann T, et al. BMC Biotechnol. 2013 Oct 4;13:8
molnova catalog



related products
-
Amyloid Bri Protein ...
Amyloid Bri Protein (1-23)
-
[Arg8]-Vasotocin TFA
[Arg8]-Vasotocin (TFA) is a nonmammalian vertebrate neurohypophyseal peptide.
-
1-(3,5-dimethoxy)phe...
1-(3',5'-dimethoxy)phenyl-2-[4''-O-β-D-glucopyranosyl (6→1)-O-α-L-rhamnopyranosyl]phenylethane showed cytotoxic activities to Hela and hep2 cell lines.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


