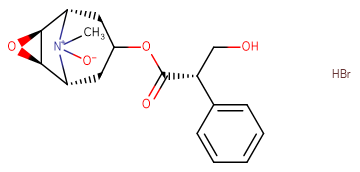
Scopolamine N-oxide HBr
CAS No. 6106-81-6
Scopolamine N-oxide HBr( —— )
Catalog No. M15295 CAS No. 6106-81-6
A muscarinic antagonist used to study binding characteristics of muscarinic cholinergic receptors.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 29 | In Stock |


|
| 10MG | 39 | In Stock |


|
| 25MG | 54 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameScopolamine N-oxide HBr
-
NoteResearch use only, not for human use.
-
Brief DescriptionA muscarinic antagonist used to study binding characteristics of muscarinic cholinergic receptors.
-
DescriptionA muscarinic antagonist used to study binding characteristics of muscarinic cholinergic receptors.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayEndocrinology/Hormones
-
TargetAChR
-
RecptormAChR| Sucrase-isomaltase
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number6106-81-6
-
Formula Weight400.26
-
Molecular FormulaC17H21NO5·HBr
-
Purity>98% (HPLC)
-
SolubilitySoluble in Water
-
SMILESC[N+]1([C@@H]2CC(C[C@H]1[C@H]3[C@@H]2O3)OC(=O)[C@H](CO)C4=CC=CC=C4)[O-].Br
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.CO TUI, DEBRUILLE C. Am J Pharm Sci Support Public Health. 1945;117:319-26.
molnova catalog



related products
-
Adiphenine hydrochlo...
Adiphenine hydrochloride is a nicotinic receptor inhibitor, used as an antispasmodic drug.
-
Clidinium bromide
Clidinium is a synthetic anticholinergic agent which has been shown in experimental and clinical studies to have a pronounced antispasmodic and antisecretory effect on the gastrointestinal tract.
-
PHA 568487
The quinuclidine PHA 568487 is an agonist of the alpha 7 nicotinic acetylcholine receptor that was designed to mitigate the bioactivation associated with the core scaffold and subsequently remove associated liabilities with in vivo tolerability.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


