PFI-3
CAS No. 1819363-80-8
PFI-3( PF-6687252 | PF-06687252 )
Catalog No. M12801 CAS No. 1819363-80-8
PFI-3 is a potent and cell active BRG/PB1 bromodomains inhibitor with Kd of 54-97 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 64 | In Stock |


|
| 5MG | 64 | In Stock |


|
| 10MG | 95 | In Stock |


|
| 25MG | 199 | In Stock |


|
| 50MG | 296 | In Stock |


|
| 100MG | 440 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NamePFI-3
-
NoteResearch use only, not for human use.
-
Brief DescriptionPFI-3 is a potent and cell active BRG/PB1 bromodomains inhibitor with Kd of 54-97 nM.
-
DescriptionPFI-3 is a potent and cell active BRG/PB1 bromodomains inhibitor with Kd of 54-97 nM; shows high selectivity over other bromodomains; exposure of embryonic stem cells to PFI-3 led to deprivation of stemness and deregulated lineage specification; markedly enhances the differentiation of trophoblast stem cells; attenuates adipocyte differentiation and dramatically decreases lipid accumulation; a first in class chemical probe for Family VIII bromodomains.
-
In VitroPFI-3 is a potent, cell-permeable probe capable of displacing ectopically expressed, GFP-tagged SMARCA2-bromodomain from chromatin. PFI-3 binds avidly to both SMARCA2 and SMARCA4 bromodomains (BROMOScan Kd's between 55 and 110 nM) consistent with the binding constant (Kd=89 nM) measured by isothermal titration calorimetry. PFI-3 does not phenocopy the growth inhibitory effects of SMARCA2 knockdown in lung cancer. Exposure of embryonic stem cells to PFI-3 leads to deprivation of stemness and deregulates lineage specification. Furthermore, differentiation of trophoblast stem cells in the presence of PFI-3 is markedly enhanced. PFI-3 binds to certain family VIII bromodomains while displaying significant, broader bromodomain family selectivity. The high specificity of PFI-3 for family VIII is achieved through a novel bromodomain binding mode of a phenolic headgroup that leads to the unusual displacement of water molecules that are generally retained by most other bromodomain inhibitors reported to date.
-
In Vivo——
-
SynonymsPF-6687252 | PF-06687252
-
PathwayChromatin/Epigenetic
-
TargetBromodomain
-
RecptorPB1(5)bromodomains|SMARCA2|SMARCA4
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number1819363-80-8
-
Formula Weight321.38
-
Molecular FormulaC19H19N3O2
-
Purity>98% (HPLC)
-
SolubilityDMSO: 32.1 mg/mL (100 mM) ( < 1 mg/ml refers to the product slightly soluble or insoluble )
-
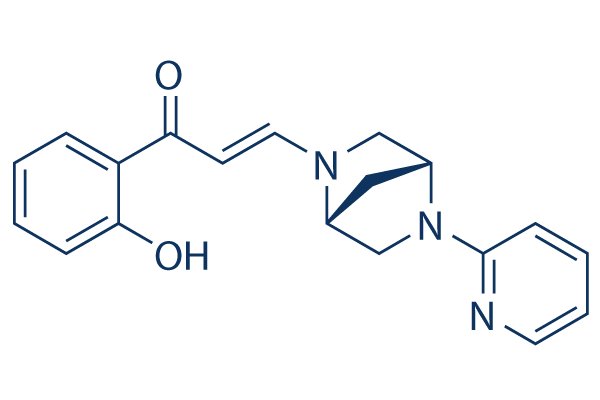
SMILESOC1=C(C(/C=C/N2[C@H](C3)CN(C4=NC=CC=C4)[C@H]3C2)=O)C=CC=C1
-
Chemical Name(E)-1-(2-hydroxyphenyl)-3-((1R,4R)-5-(pyridin-2-yl)-2,5-diazabicyclo[2.2.1]heptan-2-yl)prop-2-en-1-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Fedorov O, et al. Sci Adv. 2015 Nov 13;1(10):e1500723.
2. Vangamudi B, et al. Cancer Res. 2015 Sep 15;75(18):3865-78.
3. Gerstenberger BS, et al. J Med Chem. 2016 May 26;59(10):4800-11.
molnova catalog



related products
-
BRD-IN-26
BRD-IN-26 is a potent bromodomain inhibitor that binds the fifth bromodomain of PB1 with a KD of 124 nM.
-
LP99
LP99 is the first potent and selective BRD7/9 bromodomain inhibitor with Kd of 99 nM for BRD9; inhibits IL‐6 secretion from THP‐1 cells in a dose‐dependent manner.
-
JQ-1 carboxylic acid
JQ-1 carboxylic acid is an inhibitor of bromodomain and extra terminal domain (BET) family proteins.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


