ABT-639
CAS No. 1235560-28-7
ABT-639( ABT639 | ABT 639 )
Catalog No. M10947 CAS No. 1235560-28-7
ABT-639 is a novel, peripherally acting, selective T-type calcium channel blocker.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 55 | In Stock |


|
| 2MG | 29 | In Stock |


|
| 5MG | 42 | In Stock |


|
| 10MG | 69 | In Stock |


|
| 25MG | 163 | In Stock |


|
| 50MG | 276 | In Stock |


|
| 100MG | 458 | In Stock |


|
| 200MG | 668 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameABT-639
-
NoteResearch use only, not for human use.
-
Brief DescriptionABT-639 is a novel, peripherally acting, selective T-type calcium channel blocker.
-
DescriptionABT-639 is a novel, peripherally acting, selective T-type calcium channel blocker; blocks recombinant human Cav 3.2 in a voltage-dependent fashion (IC50=2 uM), attenuates LVA currents in rat DRG neurons (IC50=8 uM); less active at other Ca2+ channels (IC50> 30 uM); effectively reduces nociceptive and neuropathic pain in rats; high oral bioavailability.Pain Phase 2 Clinical(In Vivo):ABT-639 blocks recombinant human T-type (Cav3.2) Ca2+ channels in a voltage-dependent fashion (IC50=2 μM) and attenuates low voltage-activated (LVA) currents in rat DRG neurons (IC50=8 μM). ABT-639 is significantly less active at other Ca2+ channels (e.g. Cav1.2 and Cav2.2) (IC50>30 mM). ABT-639 has high oral bioavailability (%F=73), low protein binding (88.9%) and a low brain:plasma ratio (0.05:1) in rodents. Following oral administration ABT-639 produces dose-dependent antinociception in a rat model of knee joint pain (ED50=2 mg/kg, p.o.). ABT-639 (10-100 mg/kg, p.o.) also increases tactile allodynia thresholds in multiple models of neuropathic pain (e.g. spinal nerve ligation, CCI, and vincristine-induced, and capsaicin secondary hypersensitivity). ABT-639 does not attenuate hyperalgesia in inflammatory pain models induced by complete Freund’s adjuvant or carrageenan. At higher doses (e.g. 100-300 mg/kg) ABT-639 does not significantly alter hemodynamic or psychomotor function. The antinociceptive profile of ABT-639 provides novel insights into the role of peripheral T-type (Cav3.2) channels in chronic pain states.
-
In Vitro——
-
In VivoABT-639 blocks recombinant human T-type (Cav3.2) Ca2+ channels in a voltage-dependent fashion (IC50=2 μM) and attenuates low voltage-activated (LVA) currents in rat DRG neurons (IC50=8 μM). ABT-639 is significantly less active at other Ca2+ channels (e.g. Cav1.2 and Cav2.2) (IC50>30 mM). ABT-639 has high oral bioavailability (%F=73), low protein binding (88.9%) and a low brain:plasma ratio (0.05:1) in rodents. Following oral administration ABT-639 produces dose-dependent antinociception in a rat model of knee joint pain (ED50=2 mg/kg, p.o.). ABT-639 (10-100 mg/kg, p.o.) also increases tactile allodynia thresholds in multiple models of neuropathic pain (e.g. spinal nerve ligation, CCI, and vincristine-induced, and capsaicin secondary hypersensitivity). ABT-639 does not attenuate hyperalgesia in inflammatory pain models induced by complete Freund’s adjuvant or carrageenan. At higher doses (e.g. 100-300 mg/kg) ABT-639 does not significantly alter hemodynamic or psychomotor function. The antinociceptive profile of ABT-639 provides novel insights into the role of peripheral T-type (Cav3.2) channels in chronic pain states.
-
SynonymsABT639 | ABT 639
-
PathwayGPCR/G Protein
-
TargetCalcium Channel
-
RecptorCalciumChannel
-
Research AreaNeurological Disease
-
IndicationPain
Chemical Information
-
CAS Number1235560-28-7
-
Formula Weight455.9059
-
Molecular FormulaC20H20ClF2N3O3S
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mg/mL
-
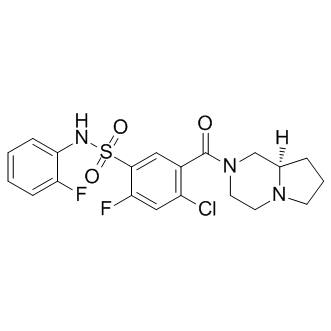
SMILESO=S(C1=CC(C(N2C[C@](CCC3)([H])N3CC2)=O)=C(Cl)C=C1F)(NC4=CC=CC=C4F)=O
-
Chemical NameBenzenesulfonamide, 4-chloro-2-fluoro-N-(2-fluorophenyl)-5-[[(8aR)-hexahydropyrrolo[1,2-a]pyrazin-2(1H)-yl]carbonyl]-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
6-Methoxyluteolin
6-Methoxyluteolin has antioxidant activity, it is a potent inhibitor of histamine release and calcium influx via down-regulation of the FcεRI α chain.Using the human basophilic KU812F cells, we assessed the inhibitory effects of 6-Methoxyluteolin, isolated from Chrysanthemum zawadskii, in the FcαμRI-mediated allergic reaction.
-
MMK 1
Potent and selective human formyl peptide receptor FPR2 agonist (EC50 values are 1, 2 and > 10 000 nM at mFRP2, hFPR2 and hFPR1 respectively). Induces migration of human monocytes and neutrophils via a chemotactic mechanism and enhances production of proinflammatory cytokines IL-1β and IL-6. Also activates the neutrophil superoxide-generating NADPH-oxidase.
-
DHBP dibromide
DHBP dibromide is calcium release and a muscle relaxant inhibitor.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


