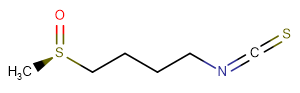
L-Sulforaphane
CAS No. 142825-10-3
L-Sulforaphane( (R)-Sulforaphane )
Catalog No. M21483 CAS No. 142825-10-3
(R)-Sulforaphane is a potent inducer of the Keap1/Nrf2/ARE pathway.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 74 | In Stock |


|
| 5MG | 123 | In Stock |


|
| 10MG | 212 | In Stock |


|
| 25MG | 357 | In Stock |


|
| 50MG | 525 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameL-Sulforaphane
-
NoteResearch use only, not for human use.
-
Brief Description(R)-Sulforaphane is a potent inducer of the Keap1/Nrf2/ARE pathway.
-
Description(R)-Sulforaphane is a potent inducer of the Keap1/Nrf2/ARE pathway.
-
In Vitro——
-
In Vivo——
-
Synonyms(R)-Sulforaphane
-
PathwayOthers
-
TargetOther Targets
-
RecptorKEAP1-Nrf2
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number142825-10-3
-
Formula Weight177.3
-
Molecular FormulaC6H11NOS2
-
Purity>98% (HPLC)
-
SolubilityDMSO:16mg/ml(90.24mM)
-
SMILESC[S@](CCCCN=C=S)=O
-
Chemical Name(R)-1-isothiocyanato-4-(methylsulfinyl)butane
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.De Nicola GR et al. Novel gram-scale production of enantiopure R-sulforaphane from Tuscan black kale seeds. Molecules. 2014 May 27;19(6):6975-86.
molnova catalog



related products
-
Neurotensin(8-13) 3T...
Neurotensin(8-13)(3TFA) is?Neurotensin (NT)?fragment, Neurotensin(8-13) results in a decrease in cell-surface NT1 receptors (NTR1) density.The internalization of the receptor induced by neurotensin (8-13) leads to a decrease in the density of the NT1 receptor (NTR1) on the cell surface.
-
N-Boc-PEG5-bromide
N-Boc-PEG5-bromide is a cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). N-Boc-PEG5-bromide is a PROTAC linker based on PEG and Alkyl/ether.
-
SJ995973
SJ995973 is a highly potent bromodomain and extraterminal (BET) protein degrader and a BET PROTAC with potential anticancer activity.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


