JNJ4796
CAS No. 2241664-16-2
JNJ4796( JNJ-4796 | JNJ 4796 )
Catalog No. M13592 CAS No. 2241664-16-2
JNJ4796 (JNJ 4796, JNJ-4796) is an orally active small-molecule fusion inhibitor of influenza virus hemagglutinin (HA) with EC50 of 33 nM (H1N1 neutralization).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 1116 | Get Quote |


|
| 50MG | 2502 | Get Quote |


|
| 100MG | 3330 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameJNJ4796
-
NoteResearch use only, not for human use.
-
Brief DescriptionJNJ4796 (JNJ 4796, JNJ-4796) is an orally active small-molecule fusion inhibitor of influenza virus hemagglutinin (HA) with EC50 of 33 nM (H1N1 neutralization).
-
DescriptionJNJ4796 (JNJ 4796, JNJ-4796) is an orally active small-molecule fusion inhibitor of influenza virus hemagglutinin (HA) with EC50 of 33 nM (H1N1 neutralization); mimics the binding and functionality of the broadly neutralizing antibody CR6261; targets the conserved HA stem region, acts as a fusion inhibitor by inhibiting conformational changes that lead to the postfusion HA structure, and neutralizes a broad spectrum of human pandemic, seasonal, and emerging group 1 influenza A viruses; specificly neutralizes influenza A group 1 viruses by inhibiting HA-mediated fusion in vitro, protects mice against lethal and sublethal influenza challenge after oral administration.
-
In VitroLike bnAb CR6261, the mechanism of action of JNJ4796 is demonstrated to be based on inhibition of the pH-sensitive conformational change of HA that triggers fusion of the viral and endosomal membranes and release of the viral genome into the host cell.
-
In VivoOral administration of JNJ4796 protects mice from lethal challenge of 25 times the median lethal dose (LD50) of H1N1 A/Puerto Rico/8/1934 virus. Doses of 50 and 10 mg/kg of JNJ4796 twice daily, initiated one day before challenge and continuing for 7 days, results in 100% survival at day 21 in comparison to the less potent compound JNJ8897 for which less than 50% survival is achieved.Oral doses of JNJ4796 results in dose-dependent efficacy after a sublethal viral challenge (LD90), with twice daily administration of 15 and 5 mg/kg of JNJ4796 giving rise to 100% survival. Animal Model:Female BALB/cAnNCrl mice intranasally infected with 2 × 25 μL of 25 × LD50 or 1 × LD90 of H1N1 A/Puerto Rico/8/34 dissolved in sterile phosphate buffered saline (D-PBS) Dosage:50 and 10 mg/kg.Administration:Oral twice daily for 7 days.Result:Resulted in 100% survival at day 21 in comparison to the less potent compound JNJ8897.
-
SynonymsJNJ-4796 | JNJ 4796
-
PathwayMicrobiology/Virology
-
TargetInfluenza Virus
-
RecptorInfluenza Virus
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2241664-16-2
-
Formula Weight537.584
-
Molecular FormulaC28H27N9O3
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (186.02 mM)
-
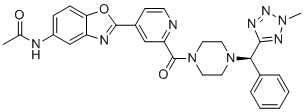
SMILESCC(NC1=CC=C(OC(C2=CC(C(N3CCN([C@@H](C4=NN(C)N=N4)C5=CC=CC=C5)CC3)=O)=NC=C2)=N6)C6=C1)=O
-
Chemical Name(R)-N-(2-(2-(4-((2-methyl-2H-tetrazol-5-yl)(phenyl)methyl)piperazine-1-carbonyl)pyridin-4-yl)benzo[d]oxazol-5-yl)acetamide
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. van Dongen MJP, et al. Science. 2019 Mar 8;363(6431). pii: eaar6221. doi: 10.1126/science.aar6221.
molnova catalog



related products
-
Dryocrassin ABBA
Dryocrassin ABBA (Dryocrassin) is a flavonoid natural product derived from Dryopteris crassirhizoma, with antiviral and antibacterial activities.
-
Baloxavir acid
Baloxavir acid (S-033447, Baloxavir) is an anti-influenza compound that potently and selectively inhibits the cap-dependent endonuclease within the polymerase PA subunit of influenza A and B viruses.
-
Molnupiravir
Molnupiravir (EIDD-2801) is an isopropyl ester prodrug of the ribonucleoside analog EIDD-1931 with oral bioavailability.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


