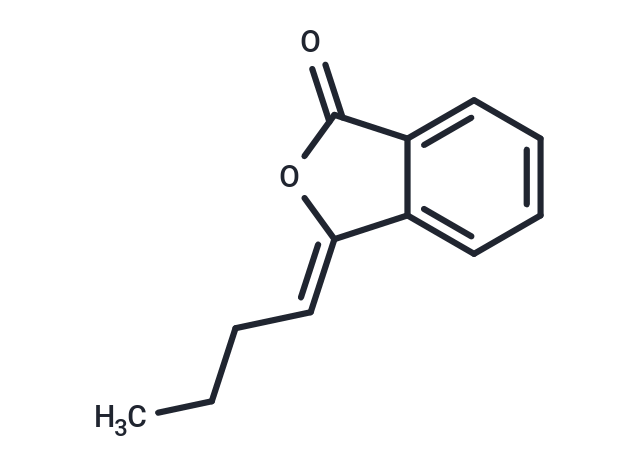
(Z)-Butylidenephthalide
CAS No. 72917-31-8
(Z)-Butylidenephthalide( —— )
Catalog No. M37746 CAS No. 72917-31-8
(Z)-Butylidenephthalide ((Z)-3-Butylidenephthalide) has antitumor and hypoglycemic effects, and can effectively inhibit the tumor growth of glioma and inhibit R-glucosidase activity.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 37 | In Stock |


|
| 5MG | 217 | In Stock |


|
| 10MG | 330 | In Stock |


|
| 25MG | 548 | In Stock |


|
| 50MG | 765 | In Stock |


|
| 100MG | 1014 | In Stock |


|
| 200MG | 1386 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name(Z)-Butylidenephthalide
-
NoteResearch use only, not for human use.
-
Brief Description(Z)-Butylidenephthalide ((Z)-3-Butylidenephthalide) has antitumor and hypoglycemic effects, and can effectively inhibit the tumor growth of glioma and inhibit R-glucosidase activity.
-
Description(Z)-3-Butylidenephthalide is an antihyperglycemic agent by inhibiting the activity of intestinal and yeast R-glucosidases (IC50=2.35 mM; Ki=4.86 mM).
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorGlucosidase
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number72917-31-8
-
Formula Weight188.22
-
Molecular FormulaC12H12O2
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESC(\CCC)=C\1/C=2C(C(=O)O1)=CC=CC2
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
Hydroxyl-γ-isosansho...
Hydroxyl-γ-isosanshool is one of the major alkylamides in Z. bungeanum and Z. schinifolium oils. Hydroxyl-γ-isosanshoo induces a numbing sensation.
-
BNP (1-32), rat TFA ...
Acts as a cardiac hormone with a variety of biological actions including natriuresis, diuresis, vasorelaxation, and inhibition of renin and aldosterone secretion. It is thought to play a key role in cardiovascular homeostasis. Helps restore the body\'s salt and water balance. Improves heart function.
-
3-O-Acetyl-16 alpha-...
3-O-Acetyl-16 alpha-hydroxytrametenolic acid has inhibitory activities against AAPH-induced hemolysis of red blood cells, it shows a strong inhibitory activity against 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


