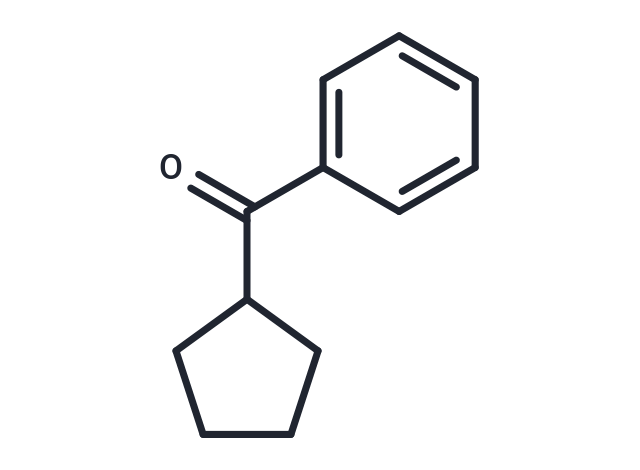
Cyclopentyl phenyl ketone
CAS No. 5422-88-8
Cyclopentyl phenyl ketone( —— )
Catalog No. M37055 CAS No. 5422-88-8
Cyclopentyl phenyl ketone (Benzoylcyclopentane) is used for the prevention and treatment of traumatic stress.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 29 | In Stock |


|
| 10MG | 35 | In Stock |


|
| 25MG | 83 | In Stock |


|
| 50MG | 118 | In Stock |


|
| 100MG | 176 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | 456 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameCyclopentyl phenyl ketone
-
NoteResearch use only, not for human use.
-
Brief DescriptionCyclopentyl phenyl ketone (Benzoylcyclopentane) is used for the prevention and treatment of traumatic stress.
-
DescriptionCyclopentyl phenyl ketone (Benzoylcyclopentane) is used for the prevention and treatment of traumatic stress.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number5422-88-8
-
Formula Weight174.24
-
Molecular FormulaC12H14O
-
Purity>98% (HPLC)
-
Solubility——
-
SMILESO=C(C1CCCC1)c1ccccc1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
Erianin
Erianin is a nature product extracted from Dendrobium chrysotoxum, has notable antitumour activity, can cause extensive tumour necrosis, growth delay.
-
Pseudo RACK1
Activator of protein kinase C; attached to cell permeabilization Antennapedia domain vector peptide. Consists of peptide derived from the C2 domain of PKC β linked by a disulfide bridge to the Antennapedia domain vector peptide. The Antennapedia peptide is actively taken up by intact cells, at 4 or 37°C, ensuring rapid and effective uptake of the activator peptide. Once inside the cell, the disulfide bonds are subjected to reduction in the cytoplasm leading to release of the activator peptide.
-
Ilexoside XLVIII
Ilexoside XLVIII is an acyl CoA cholesteryl acyl transferase ( ACAT ) inhibitor. Ilexoside XLVIII is a triterpene saponin isolated from an aqueous extract of the leaves of Ilex kudincha.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


