 Home -
Products -
Cell Cycle/DNA Damage -
Nucleoside Antimetabolite/Analog -
5-Hydroxy-2'-deoxyuridine
Home -
Products -
Cell Cycle/DNA Damage -
Nucleoside Antimetabolite/Analog -
5-Hydroxy-2'-deoxyuridine
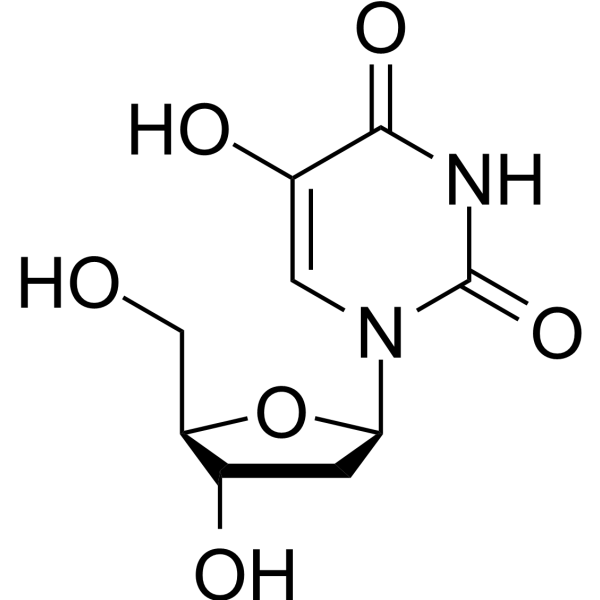
5-Hydroxy-2'-deoxyuridine
CAS No. 5168-36-5
5-Hydroxy-2'-deoxyuridine( 5-OHdU )
Catalog No. M27060 CAS No. 5168-36-5
5-Hydroxy-2'-deoxyuridine is a major oxidation product of 2'-Deoxycytidine and can be incorporated into DNA in vitro.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 50 | Get Quote |


|
| 5MG | 82 | Get Quote |


|
| 10MG | 120 | Get Quote |


|
| 25MG | 201 | Get Quote |


|
| 50MG | 300 | Get Quote |


|
| 100MG | 448 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product Name5-Hydroxy-2'-deoxyuridine
-
NoteResearch use only, not for human use.
-
Brief Description5-Hydroxy-2'-deoxyuridine is a major oxidation product of 2'-Deoxycytidine and can be incorporated into DNA in vitro.
-
Description5-Hydroxy-2'-deoxyuridine is a major oxidation product of 2'-Deoxycytidine and can be incorporated into DNA in vitro.(In Vitro):Translesion synthesis past 5-Hydroxy-2'-deoxyuridine in 18- and 45-member oligodeoxyribonucleotides occurred, but pauses both opposite, and one nucleotide prior to, the modified base in the template is observed. The specificity of nucleotide incorporation opposite 5-Hydroxy-2'-deoxyuridine in the template is sequence context-dependent. In one sequence context, dA is the principal nucleotide incorporated opposite 5-Hydroxy-2'-deoxyuridine. However, in a second sequence context, dC is the predominant base incorporated opposite 5-Hydroxy-2'-deoxyuridine. In that same sequence context, dC is also the predominant nucleotide incorporated opposite 5-Hydroxy-2'-deoxyuridine.
-
In VitroTo study the specificity of nucleotide incorporation opposite 5-hydroxypyrimidines in template DNA, 18- and 45-member oligodeoxyribonucleotides, containing an internal 2'-deoxy-5-hydroxyuridine (5-OHdU) in two different sequence contexts, were used. Translesion synthesis past 2'-deoxy-5-hydroxyuridine (5-OHdU) in both oligonucleotides occurred, but pauses both opposite, and one nucleotide prior to, the modified base in the template is observed. The specificity of nucleotide incorporation opposite 2'-deoxy-5-hydroxyuridine (5-OHdU) in the template is sequence context dependent. In one sequence context, dA is the principal nucleotide incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU). However in a second sequence context, dC is the predominant base incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU). In that same sequence context, dC is also the predominant nucleotide incorporated opposite 2'-deoxy-5-hydroxyuridine (5-OHdU).
-
In Vivo——
-
Synonyms5-OHdU
-
PathwayCell Cycle/DNA Damage
-
TargetNucleoside Antimetabolite/Analog
-
RecptorKCNQ1
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number5168-36-5
-
Formula Weight244.2
-
Molecular FormulaC9H12N2O6
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 33.33 mg/mL (136.49 mM)
-
SMILESO=C1N([C@@]2(O[C@H](CO)[C@@H](O)C2)[H])C=C(O)C(=O)N1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Mattmann ME, et al. Identification of (R)-N-(4-(4-methoxyphenyl)thiazol-2-yl)-1-tosylpiperidine-2-carboxamide, ML277, as a novel, potent and selective K(v)7.1 (KCNQ1) potassium channel activator. Bioorg Med Chem Lett. 2012;22(18):5936-5941.
molnova catalog



related products
-
2′-Deoxy-2′-fluorogu...
2′-Deoxy-2′-fluoroguanosine is a nucleoside analog that potently inhibits influenza virus A and B strains(EC90 <0.35 μM). 2'-Deoxy-2'-fluoroguanosine is an inhibitor of influenza virus replication in the upper respiratory tract, improving fever and nasal inflammation.
-
5-Azacytidine
A chemical analog of Cytidine that inhibits DNA methyltransferase; induces mitochondrial apoptosis and autophagy.
-
Enocitabine
Enocitabine is a nucleoside analog. Enocitabine inhibits the replication of human cytomegalovirus(HCMV) and it also has antileukemic and antiviral activities.



 Cart
Cart
 sales@molnova.com
sales@molnova.com

