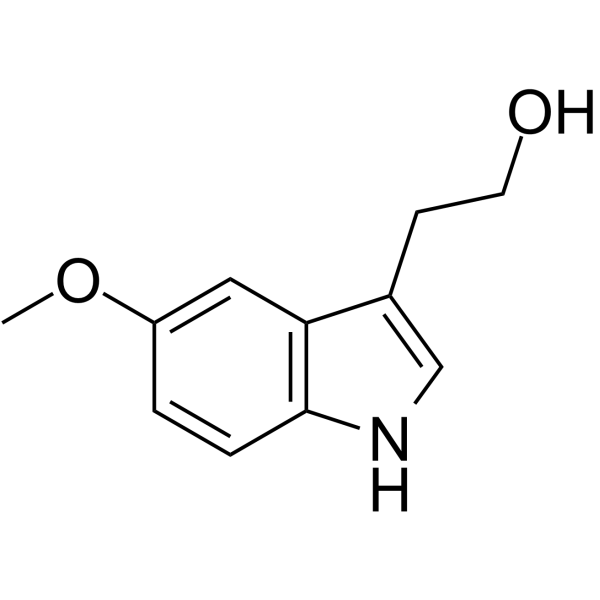
5-Methoxytryptophol
CAS No. 712-09-4
5-Methoxytryptophol( Methoxytryptophol | 2-(5-Methoxy-1H-indol-3-yl)ethanol )
Catalog No. M26544 CAS No. 712-09-4
5-Methoxytryptophol is a pineal indoleamine derived from serotonin shown to be biologically active in a number of species.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 28 | In Stock |


|
| 50MG | 39 | In Stock |


|
| 100MG | 63 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product Name5-Methoxytryptophol
-
NoteResearch use only, not for human use.
-
Brief Description5-Methoxytryptophol is a pineal indoleamine derived from serotonin shown to be biologically active in a number of species.
-
Description5-Methoxytryptophol is a pineal indoleamine derived from serotonin shown to be biologically active in a number of species.(In Vitro):In MC3T3-E1 cells, most of the 5-methoxytryptophol doses reduced slightly the metabolic activity of osteoblasts. After 14 days of cell culture, Rankl mRNA levels were decreased. 5-methoxytryptophol also induced a high osteocalcin secretion and mineralization capacity. In RAW264.7 cells, 5-methoxytryptophol decreased the number of osteoclast formed and its activity. 5-methoxytryptophol promoted activation of the ERK1/2 pathway through the phosphorylation of ERK, while LUZ addition suppressed this effect. In conclusion, 5-methoxytryptophol inhibits osteoclastogenesis and promoting osteoblast differentiation.
-
In Vitro——
-
In Vivo——
-
SynonymsMethoxytryptophol | 2-(5-Methoxy-1H-indol-3-yl)ethanol
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
RecptorNSD2-PWWP1
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number712-09-4
-
Formula Weight191.23
-
Molecular FormulaC11H13NO2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (522.93 mM)
-
SMILESCOc1ccc2[nH]cc(CCO)c2c1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
L-(-)-Malic acid
Malic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid which contributes to the sourness of a green apple. Malic acid can make a wine taste tart although the amount decreases with increasing fruit ripeness.
-
Spaglumic acid
Spaglumic acid is a neuropeptide found in millimolar concentrations in the brain.Spaglumic Acid is released upon depolarization by a Ca2+-dependent process and is an agonist at mGluR3 receptors and an antagonist at NMDA receptors.
-
1-Methylguanosine
1-Methylguanosine is a methylated nucleoside. It is known that some modified, especially methylated, nucleosides originating from RNA degradation are excreted in abnormal levels in the urine of patients with malignant tumors.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


