Stiripentol
CAS No. 49763-96-4
Stiripentol( BCX2600 )
Catalog No. M18654 CAS No. 49763-96-4
Stiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to NCLB mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively) with Ki of 1.59/0.516 μM and IC50 of 1.58/3.29 μM, respectively.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 48 | In Stock |


|
| 5MG | 45 | In Stock |


|
| 10MG | 71 | In Stock |


|
| 25MG | 145 | In Stock |


|
| 50MG | 202 | In Stock |


|
| 100MG | 341 | In Stock |


|
| 200MG | 505 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameStiripentol
-
NoteResearch use only, not for human use.
-
Brief DescriptionStiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to NCLB mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively) with Ki of 1.59/0.516 μM and IC50 of 1.58/3.29 μM, respectively.
-
DescriptionStiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to NCLB mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively) with Ki of 1.59/0.516 μM and IC50 of 1.58/3.29 μM, respectively.(In Vitro):Stiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to N-desmethylclobazam (NCLB) mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively). The inhibition of CLB demethylation by Stiripentol (STP) is best described by a noncompetitive inhibition model with apparent Ki=1.6 μM for the cDNA-expressing CYP3A4 and by a competitive inhibition model with Ki=0.52 μM for the cDNA-expressing CYP2C19. Formation of OH-NCLB from NCLB by cDNA-expressing CYP2C19 is competitively inhibited by Stiripentol (STP) with a Ki=0.14 μM.(In Vivo):In mice treating with Stiripentol (STP) monotherapy, the difference between BT1 (39.67±1.09°C) and BT2 (41.32±1.05°C) reaches statistical significance (t=3.097, p<0.05). The difference in BT2 between Stiripentol (STP) monotherapy and CLB monotherapy is statistically significant (t=2.615, p<0.05). In mice treating with Stiripentol (STP)+CLB combination therapy, the difference between BT1 (40.18±0.58°C) and BT2 (43.03±0.49°C) reaches statistical significance (t=10.44, p<0.01).
-
In VitroStiripentol (STP) is an anticonvulsant agent, which can inhibit N-demethylation of CLB to N-desmethylclobazam (NCLB) mediated by CYP3A4 (noncompetitively) and CYP2C19 (competitively). The inhibition of CLB demethylation by Stiripentol (STP) is best described by a noncompetitive inhibition model with apparent Ki=1.6 μM for the cDNA-expressing CYP3A4 and by a competitive inhibition model with Ki=0.52 μM for the cDNA-expressing CYP2C19. Formation of OH-NCLB from NCLB by cDNA-expressing CYP2C19 is competitively inhibited by Stiripentol (STP) with a Ki=0.14 μM.
-
In VivoIn mice treating with Stiripentol (STP) monotherapy, the difference between BT1 (39.67±1.09°C) and BT2 (41.32±1.05°C) reaches statistical significance (t=3.097, p<0.05). The difference in BT2 between Stiripentol (STP) monotherapy and CLB monotherapy is statistically significant (t=2.615, p<0.05). In mice treating with Stiripentol (STP)+CLB combination therapy, the difference between BT1 (40.18±0.58°C) and BT2 (43.03±0.49°C) reaches statistical significance (t=10.44, p<0.01).
-
SynonymsBCX2600
-
PathwayOthers
-
TargetOther Targets
-
RecptorCYP3A4| CYP2C19
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number49763-96-4
-
Formula Weight234.29
-
Molecular FormulaC14H18O3
-
Purity>98% (HPLC)
-
SolubilityDMSO : 150 mg/mL 640.23 mM;
-
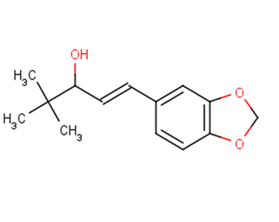
SMILESCC(C)(C)C(O)\C=C\c1ccc2OCOc2c1
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Giraud C, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism.Drug Metab Dispos. 2006 Apr;34(4):608-11. Epub 2006 Jan 13.
molnova catalog



related products
-
Aminothiazole
Aminothiazole(2-Aminothiazole) is a beginning point for synthesis of many compounds including sulfur drugs, biocides, fungicides, dyes and chemical reaction accelerators.
-
Allatotropin, Mas – ...
Allatotropin (Manse-AT) is a 13 amino acid neuropeptide. Allatotropin activates inositol 1,4,5-trisphosphate (IP3) pathway, and the biosynthesis of juvenile hormone (JH) in Manduca sexta.
-
6-Nitroveratraldehyd...
6-Nitroveratraldehyde is DNA-dependent protein kinase (DNA-PK) inhibitor with IC50 of 15 μM, an enzyme involved in the non-homologous end-joining (NHEJ) pathway of double-stranded DNA break (DSB) repair in human cells.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


