Apremilast
CAS No. 608141-41-9
Apremilast( CC 10004 | CC10004 | CC-10004 )
Catalog No. M15284 CAS No. 608141-41-9
Apremilast (CC-10004) is a potent, orally active PDE4 inhibitor with IC50 of 74 nM, inhibits TNF-α production in LPS-stimulated hPBMCs with IC50 of 77 nM.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 46 | In Stock |


|
| 5MG | 41 | In Stock |


|
| 10MG | 55 | In Stock |


|
| 25MG | 67 | In Stock |


|
| 100MG | 91 | In Stock |


|
| 200MG | 147 | In Stock |


|
| 500MG | 247 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameApremilast
-
NoteResearch use only, not for human use.
-
Brief DescriptionApremilast (CC-10004) is a potent, orally active PDE4 inhibitor with IC50 of 74 nM, inhibits TNF-α production in LPS-stimulated hPBMCs with IC50 of 77 nM.
-
DescriptionApremilast (CC-10004) is a potent, orally active PDE4 inhibitor with IC50 of 74 nM, inhibits TNF-α production in LPS-stimulated hPBMCs with IC50 of 77 nM; inhibits PBMC production of the chemokines CXCL9 and CXCL10, IFN-gamma and TNF-alpha, and IL-2, IL-12 and IL-23, also inhibits TNF-α by NK cells and keratinocytes; significantly reduces epidermal thickness and proliferation, decreases the general histopathological appearance of psoriasiform features and reduces expression of TNF-α, human leukocyte antigen-DR and intercellular adhesion molecule-1 in the lesioned skin in model of psoriasis.Psoriasis Approved(In Vitro):Apremilast (CC-10004) inhibits TNF-α release by lipopolysaccharide (LPS) with an IC50 of 104 nM (pIC50=6.98±0.2), which almost exactly replicates previous reported TNF-α inhibition by Apremilast on peripheral blood mononuclear cells (PBMCs) (IC50=110 nM) and which is similar to the potency of Apremilast for PDE4 enzymatic inhibition (IC50=74 nM). These results are clearly consistent with the hypothesis that Apremilast inhibits TNF-α by increasing intracellular cAMP levels. PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by Apremilast. (In Vivo):Apremilast (CC-10004), orally administered (5 mg/kg), significantly inhibits TNF-α production in the air pouch by 39 % (61±6 % of vehicle, P <0.001) and diminishes (by 28 %) the number of leukocytes present (72±12 % of vehicle, P<0.05). In agreement, immunohistologic analysis shows that neutrophil accumulation in the air pouch membrane is dramatically reduced by Apremilast. In the murine air pouch model, both Apremilast and methotrexate (MTX) significantly inhibit leukocyte infiltration, while Apremilast, but not MTX, significantly inhibits TNF-α release. The addition of MTX (1 mg/kg) to Apremilast (5 mg/kg) yields no more inhibition of leukocyte infiltration or TNF-α release than with Apremilast alone.Apremilast is a novel, oral PDE4 inhibitor that has been shown to regulate inflammatory mediators. After oral administration of Apremilast, a mean maximum plasma concentration (Cmax) is found to be 67.00±14.87 ng/mL. The plasma concentration of Apremilast decreases rapidly and is eliminated from plasma with a terminal half-life of 0.92±0.46 h.
-
In VitroApremilast (CC-10004) inhibits TNF-α release by lipopolysaccharide (LPS) with an IC50 of 104 nM (pIC50=6.98±0.2), which almost exactly replicates previous reported TNF-α inhibition by Apremilast on peripheral blood mononuclear cells (PBMCs) (IC50=110 nM) and which is similar to the potency of Apremilast for PDE4 enzymatic inhibition (IC50=74 nM). These results are clearly consistent with the hypothesis that Apremilast inhibits TNF-α by increasing intracellular cAMP levels. PKA, Epac1 and Epac2 knockdowns prevented TNF-α inhibition and IL-10 stimulation by Apremilast.
-
In VivoApremilast (CC-10004), orally administered (5 mg/kg), significantly inhibits TNF-α production in the air pouch by 39 % (61±6 % of vehicle, P <0.001) and diminishes (by 28 %) the number of leukocytes present (72±12 % of vehicle, P<0.05). In agreement, immunohistologic analysis shows that neutrophil accumulation in the air pouch membrane is dramatically reduced by Apremilast. In the murine air pouch model, both Apremilast and methotrexate (MTX) significantly inhibit leukocyte infiltration, while Apremilast, but not MTX, significantly inhibits TNF-α release. The addition of MTX (1 mg/kg) to Apremilast (5 mg/kg) yields no more inhibition of leukocyte infiltration or TNF-α release than with Apremilast alone.Apremilast is a novel, oral PDE4 inhibitor that has been shown to regulate inflammatory mediators. After oral administration of Apremilast, a mean maximum plasma concentration (Cmax) is found to be 67.00±14.87 ng/mL. The plasma concentration of Apremilast decreases rapidly and is eliminated from plasma with a terminal half-life of 0.92±0.46 h
-
SynonymsCC 10004 | CC10004 | CC-10004
-
PathwayAngiogenesis
-
TargetPDE
-
RecptorPDE4
-
Research AreaInflammation/Immunology
-
IndicationPsoriasis
Chemical Information
-
CAS Number608141-41-9
-
Formula Weight460.5002
-
Molecular FormulaC22H24N2O7S
-
Purity>98% (HPLC)
-
Solubility10 mM in DMSO
-
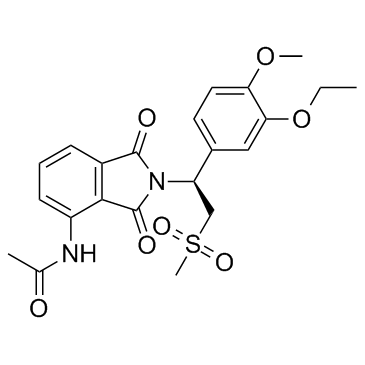
SMILESCC(NC1=CC=CC(C(N2[C@@H](C3=CC=C(OC)C(OCC)=C3)CS(=O)(C)=O)=O)=C1C2=O)=O
-
Chemical NameAcetamide, N-[2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]-
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Man HW, et al. J Med Chem. 2009 Mar 26;52(6):1522-4.
2. McCann FE, et al. Arthritis Res Ther. 2010;12(3):R107.
3. Schafer PH, et al. Br J Pharmacol. 2010 Feb;159(4):842-55.
4. Gordon JN, et al. J Crohns Colitis. 2009 Sep;3(3):175-82.
molnova catalog



related products
-
PF 05180999
PF-05180999 (PF 5180999) is a potent, selective, brain-penetrating and orally bioavailable phosphodiesterase 2A (PDE2A) inhibitor with IC50 of 1 nM.
-
N-Methylbenzamide
N-Methylbenzamide is a potent phosphodiesterase 10A (PDE10A) inhibitor,with anti-cancer activity.
-
Imazodan hydrochlori...
Imazodan hydrochloride (CI-914 HCl) is a potent and selective type III phosphodiesterase inhibitor used in the treatment of chronic congestive heart failure.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


