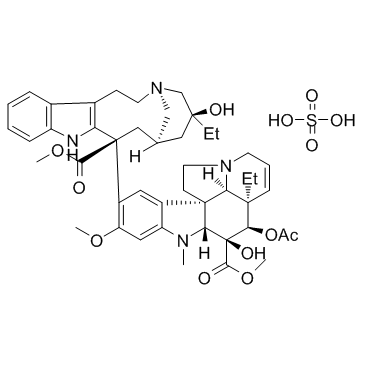
Vinblastine sulfate
CAS No. 143-67-9
Vinblastine sulfate( 29060LE | Alkaban-AQ | Exal | NSC 49842 | Rozevinsulfate | Velban | Velsar )
Catalog No. M11857 CAS No. 143-67-9
Vinblastine sulfate can inhibit the formation of microtubule, it also inhibit nAChR(IC50=8.9 uM).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 1 mL x 10 mM in DMSO | 60 | In Stock |


|
| 5MG | 48 | In Stock |


|
| 10MG | 51 | In Stock |


|
| 25MG | 61 | In Stock |


|
| 50MG | 78 | In Stock |


|
| 100MG | 90 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameVinblastine sulfate
-
NoteResearch use only, not for human use.
-
Brief DescriptionVinblastine sulfate can inhibit the formation of microtubule, it also inhibit nAChR(IC50=8.9 uM).
-
DescriptionVinblastine sulfate can inhibit the formation of microtubule, it also inhibit nAChR(IC50=8.9 uM).(In Vitro):Vinblastine does not depolymerize spindle microtubules, yet it powerfully blocks mitosis (for example, IC50 0.8 nM in HeLa cells) and cells die by apoptosis. In NB4 cells, vinblastine produces alteration of p53 and DNA fragmentation. Vinblastine treatment has an antiproliferative effect via the induction of apoptosis producing Bax/Bcl-2 imbalance. Vinblastine treatment suppresses NFκB expression and depresses NFκB-DNA binding activity while maintaining JNK activation that subsequently results in apoptotic response through caspase-dependent pathway. Vinblastine is found to trigger apoptosis as evidenced by the loss of mitochondrial membrane potential, the release of both cytochrome c and apoptosis inducing factor, activation of caspase-9 and 3, and cleavage of Poly (ADP-ribose)-Polymerase.(In Vivo):Vinblastine is a widely used anticancer drug with undesired side effects. Its conjugation with carrier molecules could be an efficient strategy to reduce these side effects.
-
In VitroVinblastine does not depolymerize spindle microtubules, yet it powerfully blocks mitosis (for example, IC50 0.8 nM in HeLa cells) and cells die by apoptosis. In NB4 cells, vinblastine produces alteration of p53 and DNA fragmentation. Vinblastine treatment has an antiproliferative effect via the induction of apoptosis producing Bax/Bcl-2 imbalance. Vinblastine treatment suppresses NFκB expression and depresses NFκB-DNA binding activity while maintaining JNK activation that subsequently results in apoptotic response through caspase-dependent pathway. Vinblastine is found to trigger apoptosis as evidenced by the loss of mitochondrial membrane potential, the release of both cytochrome c and apoptosis inducing factor, activation of caspase-9 and 3, and cleavage of Poly (ADP-ribose)-Polymerase.
-
In VivoVinblastine is a widely used anticancer drug with undesired side effects. Its conjugation with carrier molecules could be an efficient strategy to reduce these side effects.
-
Synonyms29060LE | Alkaban-AQ | Exal | NSC 49842 | Rozevinsulfate | Velban | Velsar
-
PathwayEndocrinology/Hormones
-
TargetAChR
-
RecptornAChR
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number143-67-9
-
Formula Weight909.06
-
Molecular FormulaC46H58N4O9·H2SO4
-
Purity>98% (HPLC)
-
SolubilitySoluble in DMSO
-
SMILESCC[C@@]1(C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)[C@]78CCN9[C@H]7[C@@](C=CC9)([C@H]([C@@]([C@@H]8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.OS(=O)(=O)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Gigant B, et al. Nature, 2005, 435(7041), 519-522.
molnova catalog



related products
-
Loxeen
Pridinol Methanesulfonate Salt is used as an anticholinergic and as an antiparkinsonian agent.
-
Homatropine Bromide
Homatropine Bromide is muscarinic AChR antagonist, inhibits endothelial and smooth muscle muscarinic receptors.
-
Oxybutynin hydrochlo...
Oxybutynin is also a possible treatment of hyperhidrosis (hyper-active sweating) .



 Cart
Cart
 sales@molnova.com
sales@molnova.com


