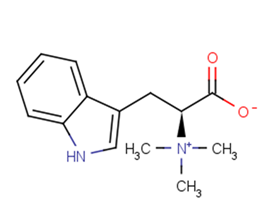
Hypaphorine
CAS No. 487-58-1
Hypaphorine( Hypaphorine | Hypaforin | L-Hypaphorine )
Catalog No. M18635 CAS No. 487-58-1
Hypaphorine is an indole-3-acetic acid antagonist which specifically compete with indole-3-acetic acid in binding to the indole-3-acetic acid-binding site in plant peroxidases.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 248 | In Stock |


|
| 10MG | 419 | In Stock |


|
| 25MG | 689 | In Stock |


|
| 50MG | 963 | In Stock |


|
| 100MG | 1305 | In Stock |


|
| 500MG | 2592 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameHypaphorine
-
NoteResearch use only, not for human use.
-
Brief DescriptionHypaphorine is an indole-3-acetic acid antagonist which specifically compete with indole-3-acetic acid in binding to the indole-3-acetic acid-binding site in plant peroxidases.
-
DescriptionHypaphorine is an indole alkaloid, present in the fungus Pisolithus tinctorius in family Sclerodermataceae, that controls the elongation rate of root hairs.
-
In Vitro——
-
In Vivo——
-
SynonymsHypaphorine | Hypaforin | L-Hypaphorine
-
PathwayEndocrinology/Hormones
-
TargetAChR
-
RecptorOthers
-
Research AreaMetabolic Disease
-
Indication——
Chemical Information
-
CAS Number487-58-1
-
Formula Weight246.31
-
Molecular FormulaC14H18N2O2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?H2O : 100 mg/mL (406.01 mM)
-
SMILESC[N+](C)(C)C(CC1=CNC2=CC=CC=C21)C(=O)[O-]
-
Chemical Name(S)-3-(1H-indol-3-yl)-2-(trimethylammonio)propanoate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
TTNPB
TTNPB, a potent RAR agonist, inhibits binding of [3H]tRA of human RARα (IC50: 5.1 nM), β (IC50: 4.5 nM), and γ (IC50: 9.3 nM), respectively.
-
Clothianidin
Clothianidin, an insecticide, acts as an agonist of acetylcholine to stimulate nAChR, thereby activating post-synaptic acetylcholine receptors but not inhibiting AChE.
-
3-Epioleanolic acid
3-Epioleanolic acid and oleanonic acid possess varying degrees of agonist activity on uterine smooth muscle with minor changes in the molecular structure affecting its intrinsic activity on uterine muscle.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


