Glafenine
CAS No. 3820-67-5
Glafenine( Glafenine | Glafenin | Glafenina )
Catalog No. M14324 CAS No. 3820-67-5
Glafenine is a non-steroidal anti-inflammatory drug (NSAID), is a non-narcotic analgesic agent, widely used for the treatment of pains of various origins..
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 32 | In Stock |


|
| 100MG | 45 | In Stock |


|
| 200MG | 61 | In Stock |


|
| 500MG | 133 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameGlafenine
-
NoteResearch use only, not for human use.
-
Brief DescriptionGlafenine is a non-steroidal anti-inflammatory drug (NSAID), is a non-narcotic analgesic agent, widely used for the treatment of pains of various origins..
-
DescriptionGlafenine is a non-steroidal anti-inflammatory drug (NSAID), is a non-narcotic analgesic agent, widely used for the treatment of pains of various origins.
-
In Vitro——
-
In Vivo——
-
SynonymsGlafenine | Glafenin | Glafenina
-
PathwayChromatin/Epigenetic
-
TargetCOX
-
RecptorCOX
-
Research AreaInflammation/Immunology
-
Indication——
Chemical Information
-
CAS Number3820-67-5
-
Formula Weight372.8
-
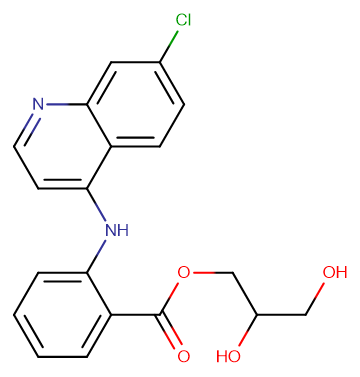
Molecular FormulaC19H17ClN2O4
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESO=C(OCC(O)CO)C1=CC=CC=C1NC2=CC=NC3=CC(Cl)=CC=C23
-
Chemical Name2,3-Dihydroxypropyl N-(7-chloro-4-quinolyl) anthranilate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Kleinknecht D, et al. Clin Nephrol. 1986 Jun;25(6):275-8
molnova catalog



related products
-
Firocoxib
Firocoxib is a selective non-steroidal inhibitor of cycooxygenase-2 (COX-2) (IC50 of 0.13 μM), with anti-inflammatory for use in dogs and horses.
-
8-SHOGAOL
8-?Shogaol is a component of ginger and maintains anti-inflammatory activity as a cyclooxygenase-2 inhibitor.
-
Valdecoxib
Valdecoxib was removed from the Canadian, U.S., and E.U. markets in 2005 due to concerns about possible increased risk of heart attack and stroke.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


