Anandamide
CAS No. 94421-68-8
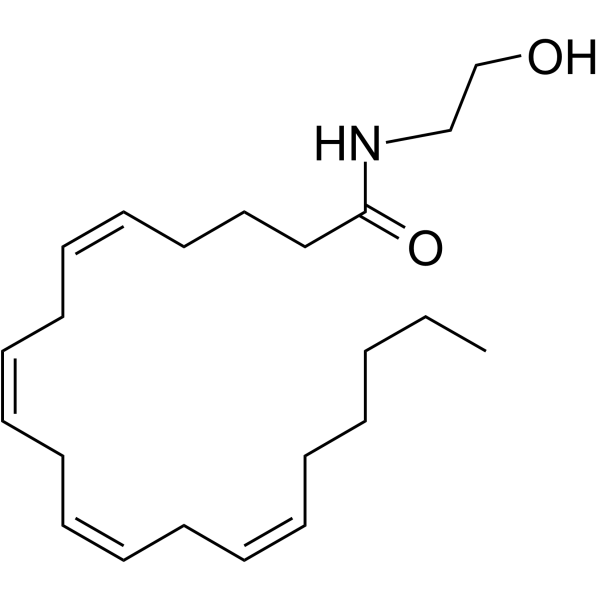
Anandamide( (5Z,8Z,11Z,14Z)-N-(2-Hydroxyethyl)icosa-5,8,11,14-tetraenamide )
Catalog No. M26593 CAS No. 94421-68-8
Anandamide, an immune modulator, acts via not only cannabinoid receptors (CB1 and CB2) but also other targets (e.g., GPR18/GPR55) in the central nervous system.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 48 | Get Quote |


|
| 5MG | 71 | Get Quote |


|
| 10MG | 141 | Get Quote |


|
| 25MG | 267 | Get Quote |


|
| 50MG | 412 | Get Quote |


|
| 100MG | 606 | Get Quote |


|
| 500MG | 1287 | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameAnandamide
-
NoteResearch use only, not for human use.
-
Brief DescriptionAnandamide, an immune modulator, acts via not only cannabinoid receptors (CB1 and CB2) but also other targets (e.g., GPR18/GPR55) in the central nervous system.
-
DescriptionAnandamide, an immune modulator, acts via not only cannabinoid receptors (CB1 and CB2) but also other targets (e.g., GPR18/GPR55) in the central nervous system.(In Vitro):Anandamide, acting via CB2 receptors, alleviates lipopolysaccharide (LPS)-induced neuroinflammation in rat primary microglial cultures. Endocannabinoids, especially Anandamide (AEA), can activate numerous other receptors like PPARS, TRPV1, and GPR18/GPR55 .(In Vivo):Anandamide is an endocannabinoid binding both CB1R and CB2R. To evaluate the impact of CBR activation on whole-body glucose homeostasis, glucose tolerance is assessed after a single intraperitoneal Anandamide injection (10 mg/kg). The increase in glycemia in response to glucose ingestion is considerably smaller in mice treated with Anandamide compared with control. It is associated with an improvement of glucose tolerance as illustrated by the AUC0-2h calculations.
-
In VitroAnandamide (0-250 μg/ml, 1 h) inhibits C. albicans hyphal growth and prevents hyphal adherence to epithelial cells.Anandamide (0-250 μg/ml, 2 h) alters the expression of genes involved in adhesion and hyphal morphogenesis.Anandamide reduces tau phosphorylation through the inhibition of the activity of protein kinases. RT-PCRCell Line:HeLa cervical epithelial cells Concentration:0, 10, 50, 125, 250 μg/ml Incubation Time:2 h Result:Repressed the expression of the HWP1 and ALS3 adhesins involved in Candida adhesion to epithelial cells and the HGC1, RAS1, EFG1 and ZAP1 regulators of hyphal morphogenesis and cell adherence. Increased the expression of NRG1, a transcriptional repressor of filamentous growth.
-
In VivoAnandamide (10 mg/kg, IP, once) considerably lowers the increase of glycemia in response to glucose ingestion compared with control, and this is associated with an improvement of glucose tolerance.Anandamide (100 ng, ICV bilateral injection, single) partially prevents streptozotocin (STZ)-induced cognitive impairments, changes in synaptic markers and ventricle enlargement.Anandamide exerts anti-inflammatory activities, attenuating the development of inflammation in a mouse model of ulcerative colitis.Anandamide alleviates lipopolysaccharide (LPS)-induced neuroinflammation in rat primary microglial cultures. Animal Model:Male Wistar rats (3-4 months, 350-400 g, STZ-induced AD-like sporadic dementia model) Dosage:100 ng Administration:ICV bilateral injection, single Result:Prevented recognition and non-associative emotional memory impairments induced by STZ, but did not influence tone fear conditioning. Decreased BAG2 (Bcl2-associated athanogene) levels, and prevented STZ-induced ventricle enlargement.
-
Synonyms(5Z,8Z,11Z,14Z)-N-(2-Hydroxyethyl)icosa-5,8,11,14-tetraenamide
-
PathwayGPCR/G Protein
-
TargetCannabinoid Receptor
-
Recptor——
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number94421-68-8
-
Formula Weight347.53
-
Molecular FormulaC22H37NO2
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : ≥ 100 mg/mL (287.74 mM)
-
SMILESCCCCC/C=C\C/C=C\C/C=C\C/C=C\CCCC(NCCO)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Motoi Yamashita, et al. The effect of pressure on the phase transition behavior of tridecane, pentadecane, and heptadecane: a Fourier transform infrared spectroscopy study. J Chem Phys. 2011 Apr 14;134(14):144503.
molnova catalog



related products
-
AM-2201
A synthetic cannabinoid that acts as a potent, nonselective full agonist for the cannabinoid receptor with EC50 of 38 and 58 nM for CB1 and CB2, respectively.
-
LDK1229
LDK1229 is a novel potent, selective cannabinoid CB1 receptor inverse agonist with Ki of 220 nM.
-
CB1 antagonist 2
CB1 antagonist 2 is caimabinoid 1 (CB1) antagonist extracted from patent WO2016184310A1, compound 3. Which inhibits CB1 in vivo with an IC50 of 25.5 nM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


