PF-8380
CAS No. 1144035-53-9
PF-8380( PF8380 | PF-8380 | PF 8380 )
Catalog No. M10512 CAS No. 1144035-53-9
Potent autotaxin inhibitor (IC50?= 2.8 nM in isolated enzyme assay; 101 nM in human whole blood).
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 35 | In Stock |


|
| 10MG | 58 | In Stock |


|
| 25MG | 125 | In Stock |


|
| 50MG | 205 | In Stock |


|
| 100MG | 335 | In Stock |


|
| 200MG | 494 | In Stock |


|
| 500MG | 782 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NamePF-8380
-
NoteResearch use only, not for human use.
-
Brief DescriptionPotent autotaxin inhibitor (IC50?= 2.8 nM in isolated enzyme assay; 101 nM in human whole blood).
-
DescriptionPotent autotaxin inhibitor (IC50?= 2.8 nM in isolated enzyme assay; 101 nM in human whole blood). Modulates lysophosphatidic acid (LPA) levels?in vivo?and?in vitro?by directly inhibiting autotaxin; reduces LPA levels both in plasma and at site of inflammation. Orally available.
-
In VitroPF-8380 also inhibits rat autotaxin with an IC50 of 1.16 nM with FS-3 substrate. Potency of PF-8380 is maintained when using enzyme produced from fetal fibroblasts used in combination with lysophosphatidyl choline (LPC) as a substrate. In human whole blood incubated with PF-8380 for 2 h, autotaxin is inhibited with an IC50 of 101 nM. Autotaxin (ATX), an enzyme with lysophospholipase D (lysoPLD) activity, catalyzes the production of lysophosphatidic acid (LPA) from lysophosphatidylcholine (LPC). Pre-treatment of GL261 and U87-MG cells with 1 μM PF-8380 followed by 4 Gy irradiation results in decreased clonogenic survival, decreases migration (33% in GL261; P=0.002 and 17.9% in U87-MG; P=0.012), decreases invasion (35.6% in GL261; P=0.0037 and 31.8% in U87-MG; P=0.002), and attenuates radiation-induced Akt phosphorylation.
-
In VivoThe pharmacokinetic profile of PF-8380 is evaluated at an intravenous dose of 1 mg/kg and oral doses of 1 to 100 mg/kg out to 24 h. PF-8380 has mean clearance of 31 mL/min/kg, volume of distribution at steady state of 3.2 L/kg, and effective t1/2 of 1.2 h. Oral bioavailability is moderate, ranging from 43 to 83%. Plasma concentrations increased with single oral escalating doses, but Cmax increased at a rate that is approximately proportional to dose from 1 to 10 mg/kg and less than proportional to dose from 10 to 100 mg/kg. PF-8380 exposures estimated by area under the curve are approximately proportional to dose and linear up to 100 mg/kg. Plasma C16:0, C18:0, and C20:0 LPA levels are measured immediately after collection. Maximal reduction of LPA levels is observed by the 3 mg/kg dose at 0.5 h with all LPA returning at or above baseline at 24 h. Treatment with 10 mg/kg PF-8380 increases tumor-associated vascularity modestly by 20% (P=0.497). When compared to control, treatment of PF-8380 45 min before 4 Gy irradiation decreases vascularity by nearly 48% when compared to control (P=0.031) and by 65% when compared to mice that received radiation alone (P=0.011).
-
SynonymsPF8380 | PF-8380 | PF 8380
-
PathwayAngiogenesis
-
TargetPDE
-
RecptorAutotaxin
-
Research AreaInflammation/Immunology
-
Indication——
Chemical Information
-
CAS Number1144035-53-9
-
Formula Weight478.33
-
Molecular FormulaC22H21Cl2N3O5
-
Purity>98% (HPLC)
-
SolubilityDMSO: 95 mg/mL (198.6 mM)
-
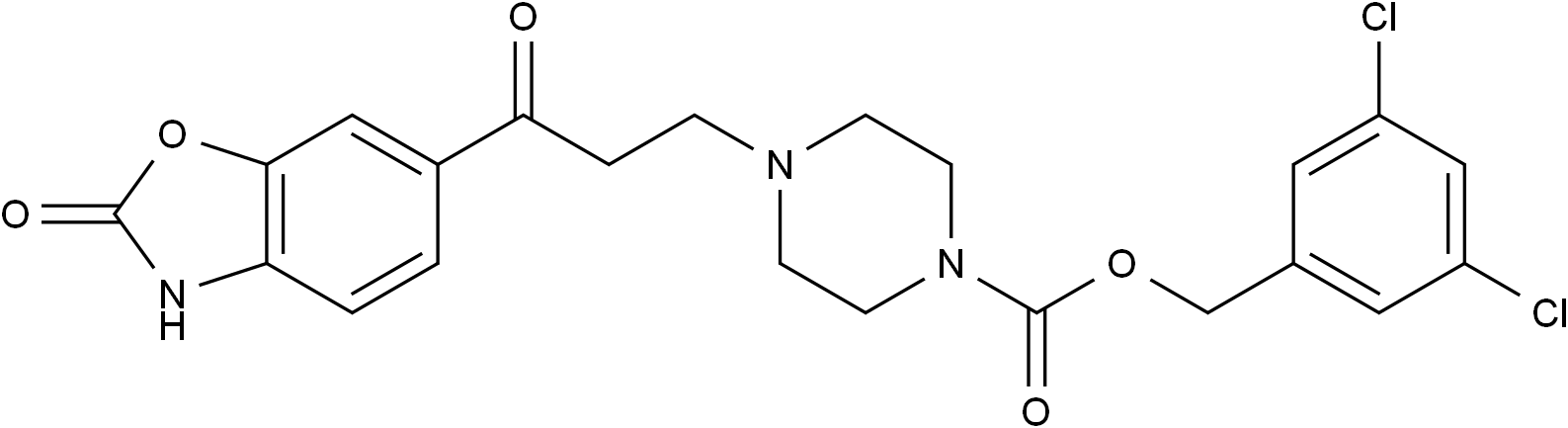
SMILESO=C(N1CCN(CCC(C2=CC=C3NC(OC3=C2)=O)=O)CC1)OCC4=CC(Cl)=CC(Cl)=C4
-
Chemical Name3,5-dichlorobenzyl 4-(3-oxo-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-6-yl)propyl)piperazine-1-carboxylate
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Gierse J, et al. J Pharmacol Exp Ther. 2010, 334(1):310-317.
molnova catalog



related products
-
AZD1283
AZD1283 is an effective P2Y12 receptor antagonist (EC50: 3.0 ug/kg/min, binding IC50: 11 nM).
-
N-Ethyl tadalafil
N-Ethyl tadalafil (NEthyl tadalafil) is a CGMP-specific 3',5'-cyclic phosphodiesterase (PDE) inhibitor used in the study of cardiovascular diseases such as angina pectoris, hypertension, and pulmonary hypertension.
-
Vardenafil hydrochlo...
Vardenafil hydrochloride is a New Phosphodiesterase Type 5(PDE5) Inhibitor in the Treatment of Erectile Dysfunction in Men With Diabetes.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


